��Ŀ����
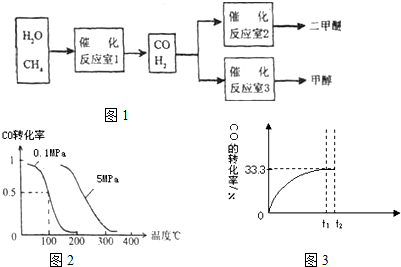
�״�����Ϊ21���͵�����ȼ�ϣ���ҵ��ͨ�����з�Ӧ�ٺ͢ڣ���CH4��H2OΪԭ�����Ʊ��״���
��CH4(g)+H2O(g) CO(g)+3H2(g) ��H1
CO(g)+3H2(g) ��H1
��CO(g)+2H2(g) CH3OH(g) ��H2
CH3OH(g) ��H2
��0.20 mol CH4��0.30 mol H2O(g)ͨ���ݻ�Ϊ10L���ܱ������У���һ�������·�����Ӧ�٣��ﵽƽ��ʱ��CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��
��CH4(g)+H2O(g)
 CO(g)+3H2(g) ��H1
CO(g)+3H2(g) ��H1 ��CO(g)+2H2(g)
 CH3OH(g) ��H2
CH3OH(g) ��H2 ��0.20 mol CH4��0.30 mol H2O(g)ͨ���ݻ�Ϊ10L���ܱ������У���һ�������·�����Ӧ�٣��ﵽƽ��ʱ��CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��

(1)�¶Ȳ��䣬��С���������ѹǿ���ٵķ�Ӧ����_______����� ����С���� �䡱����
ƽ����________ �ƶ���
(2)��Ӧ�ٵġ�H1__���<����=����>����0����ƽ�ⳣ������ʽΪK=_________��100��ʱ��ƽ�ⳣ��ֵ��_________
(3)��ѹǿΪ0.1 MPa�����£���a mol CO��3a mol H2�Ļ�������ڴ��������½��з�Ӧ�����ɼ״���
Ϊ��Ѱ�Һϳɼ״����¶Ⱥ�ѹǿ������������ijͬѧ���������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��С������±��ո�������ʣ���ʵ���������ݡ�
ƽ����________ �ƶ���
(2)��Ӧ�ٵġ�H1__���<����=����>����0����ƽ�ⳣ������ʽΪK=_________��100��ʱ��ƽ�ⳣ��ֵ��_________
(3)��ѹǿΪ0.1 MPa�����£���a mol CO��3a mol H2�Ļ�������ڴ��������½��з�Ӧ�����ɼ״���
Ϊ��Ѱ�Һϳɼ״����¶Ⱥ�ѹǿ������������ijͬѧ���������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��С������±��ո�������ʣ���ʵ���������ݡ�

(1)�����淴Ӧ�������
(2)>�� ��
��
(2)>��
 ��
��
(3)


��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
�����Ŀ
 ��2012?����ģ�⣩��Դ��ȱ���������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������˼״�����Ϊ21���͵�����ȼ�ϣ�
��2012?����ģ�⣩��Դ��ȱ���������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������˼״�����Ϊ21���͵�����ȼ�ϣ�