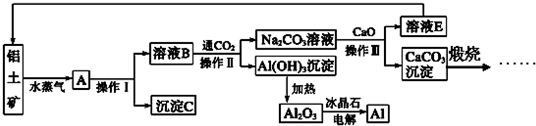
��Ŀ����
��14�֣���ش��������⣺
��1�����������Ӧ�������ԾǨ�ص���________________________________��
A������ B����ɫ��Ӧ C��ȼ�շ��� D��ԭ�ӹ��� E��ʯī����
��2��A��B���ֶ�����Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�Bԭ�������������Ǵ�����������ijƽ���������η�����A��B����Ԫ�������ԭ�Ӹ�����Ϊ1��1���÷����к���_______���Ҽ���
��3��Ԫ�ظ�������(CrO2Cl2)���л��ϳ��п������������Ȼ��������������л��ﷴӦ��
�����ͬ���ڵĻ�̬ԭ�����������������ԭ����ͬ��Ԫ����______����Ԫ�ط��ţ���
����a:�� b:CH3OH c:HCHO d:CS2 e:CCl4 �����л��ܼ��У�̼ԭ�Ӳ�ȡsp3�ӻ��ķ�����___________������ĸ����CS2 ���ӵļ�����__________��
���ӵļ�����__________��
�۹��ɽ���������ˮ�����γɵ�������Ƿ�����ɫ������d��������Ų��йء�һ��أ�Ϊd0��d10�Ų�ʱ������ɫ��Ϊd1��d9�Ų�ʱ������ɫ���� [Co(H2O)6]2+�Էۺ�ɫ������Co2+�ļ۵����Ų�ʽΪ________________________
[Co(H2O)6]2+�Էۺ�ɫ������Co2+�ļ۵����Ų�ʽΪ________________________
(4)����CO���Ժϳɻ���ԭ��COCl2������
��COCl2���ӵĽṹʽΪ ����COCl2�����ں��� �����ţ���
����COCl2�����ں��� �����ţ���
���� A��4���Ҽ� B��2���Ҽ���2���м�
C��2���Ҽ���1���м� D��3���Ҽ���1���м�
��14�֣�(1)C �� E ��2�֣�(2) 12 ��2�֣�
(3) �� Cu K ��2�֣��� b e ��2�֣� 1800 ��2�֣��� 3d7 ��2�֣�
(4) D ��2�֣�
����

 ��У����ϵ�д�
��У����ϵ�д�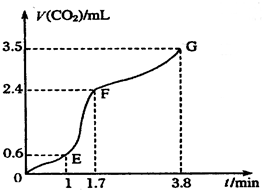 ��������̼��ƹ����ϡ���ᷴӦ��ȡCO2���壬��ش��������⣺
��������̼��ƹ����ϡ���ᷴӦ��ȡCO2���壬��ش��������⣺