��Ŀ����
�������Ļ�����Ӧ�ù㷺����FeCl3������������ӡˢ��·ͭ�帯ʴ��������ֹѪ���ȡ�
(1)д��FeCl3��Һ��ʴӡˢ��·ͭ������ӷ���ʽ____________________________________________��
(2)����(1)�еķ�Ӧ��Ƴ�ԭ��أ��뻭��ԭ��ص�װ��ͼ�����������������д���缫��Ӧʽ��
ͼ��______________������Ӧ��___________________��������Ӧ��__________________��
(3)��ʴͭ���Ļ����Һ�У���Cu2+��Fe3+��Fe2+��Ũ�Ⱦ�Ϊ0.10 mol��L-1��������±����������ݺ�ҩƷ��������ȥCuCl2��Һ��Fe3+��Fe2+��ʵ�鲽��____________________________________��
(1)д��FeCl3��Һ��ʴӡˢ��·ͭ������ӷ���ʽ____________________________________________��
(2)����(1)�еķ�Ӧ��Ƴ�ԭ��أ��뻭��ԭ��ص�װ��ͼ�����������������д���缫��Ӧʽ��
ͼ��______________������Ӧ��___________________��������Ӧ��__________________��
(3)��ʴͭ���Ļ����Һ�У���Cu2+��Fe3+��Fe2+��Ũ�Ⱦ�Ϊ0.10 mol��L-1��������±����������ݺ�ҩƷ��������ȥCuCl2��Һ��Fe3+��Fe2+��ʵ�鲽��____________________________________��

(4)ij������Ա�������ʲ���������и�ʴ����������ijЩ����Һ�и�ʴ�������ԡ�����ϱ��ṩ��ҩƷ��ѡ������(ˮ����ѡ)��������ʵ�飬��֤���ʲ�����ױ���ʴ���йط�Ӧ�Ļ�ѧ����ʽ
________________________��________________________�����ʲ���ָ�ʴ��ʵ������________________________________________��
________________________��________________________�����ʲ���ָ�ʴ��ʵ������________________________________________��
(1)2Fe3+��Cu = 2Fe2+��Cu2+
(2)װ��ͼ��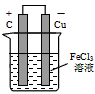 ��������Ӧ��Fe3+��e- = Fe2+(��2Fe3+��2e-==2Fe2+)��������Ӧ��
��������Ӧ��Fe3+��e- = Fe2+(��2Fe3+��2e-==2Fe2+)��������Ӧ��
Cu��2e-=Cu2+
(3)��ͨ������������Fe2+������Fe3+��
�ڼ���CuO������Һ��pH��3.2��4.7��
�۹���[��ȥFe(OH)3]
(4)CuO��H2SO4==CuSO4��H2O��CuSO4��Fe==FeSO4��Cu������ֱ������Ϻ�ɫ��������
(2)װ��ͼ��
 ��������Ӧ��Fe3+��e- = Fe2+(��2Fe3+��2e-==2Fe2+)��������Ӧ��
��������Ӧ��Fe3+��e- = Fe2+(��2Fe3+��2e-==2Fe2+)��������Ӧ��Cu��2e-=Cu2+
(3)��ͨ������������Fe2+������Fe3+��
�ڼ���CuO������Һ��pH��3.2��4.7��
�۹���[��ȥFe(OH)3]
(4)CuO��H2SO4==CuSO4��H2O��CuSO4��Fe==FeSO4��Cu������ֱ������Ϻ�ɫ��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ