��Ŀ����
SO2�Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ������SO2�� ������ijѧ������С�����������ͼ��ʾʵ��װ�á�
������ijѧ������С�����������ͼ��ʾʵ��װ�á�
���Թ��м���0.0005mol?L��1��ˮ1.0mL��������������ˮϡ�ͺ��ټ���2��3�ε�����Һ�����Ƴ���ҺA���ⶨָ���ص�Ŀ�����SO2�ĺ���ʱ������ע�����Ļ�������������A��Һ�� ɫ��Ϊ ɫʱ��Ӧǡ����ȫ���У���ʱֹͣ������
A���ɺ�ɫ��Ϊ��ɫ B������ɫ��Ϊ��ɫ
C������ɫ��Ϊ��ɫ D������ɫ��Ϊ��ɫ
C

��ϰ��ϵ�д�
�����Ŀ

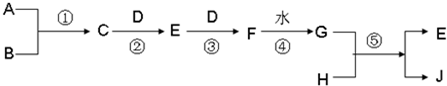

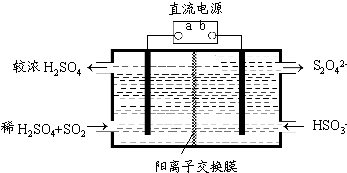
 7N2(g)+12H2O(g)+Q��Q��0����
7N2(g)+12H2O(g)+Q��Q��0����