��Ŀ����
(16��)���仯����������ת����ϵ
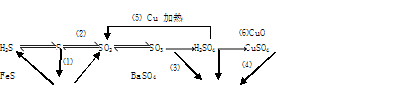
(1)����������ԭ��Ӧ���� (�����)
(2)д��(5)�ķ�Ӧ����ʽ ��
˵��Ũ������� ����ȡCuSO4�� �������(�����)��
(3)SO2����ɿ�����Ⱦ���γ��������Ҫ���ʡ�SO2��ˮ�Ĵ�������������Ӧ�������ᣬ��Ӧ����ʽΪ ��
��֤��������ķ����� ��
(4)ij��Һ�к���Cl-��SO42-�����ܺ���Na+��Fe2+������һ�֡�
����֤Cl-��SO42-�ķ�����
A.�ȼ�BaCl2��Һ���ȳ������ټ�AgNO3��Һ
B.�ȼ�AgNO3��Һ���ȳ������ټ�BaCl2��Һ
C.�ȼ�Ba(NO3)2��Һ���ȳ������ټ�AgNO3��Һ
����֤Na+��Fe2+��ķ����� ��

(1)����������ԭ��Ӧ���� (�����)
(2)д��(5)�ķ�Ӧ����ʽ ��
˵��Ũ������� ����ȡCuSO4�� �������(�����)��
(3)SO2����ɿ�����Ⱦ���γ��������Ҫ���ʡ�SO2��ˮ�Ĵ�������������Ӧ�������ᣬ��Ӧ����ʽΪ ��
��֤��������ķ����� ��
(4)ij��Һ�к���Cl-��SO42-�����ܺ���Na+��Fe2+������һ�֡�
����֤Cl-��SO42-�ķ�����
A.�ȼ�BaCl2��Һ���ȳ������ټ�AgNO3��Һ
B.�ȼ�AgNO3��Һ���ȳ������ټ�BaCl2��Һ
C.�ȼ�Ba(NO3)2��Һ���ȳ������ټ�AgNO3��Һ
����֤Na+��Fe2+��ķ����� ��
(16��)
(1) (1)(2)(5) (2��)
(2)2H2SO4(Ũ)+Cu CuSO4+SO2��+2H2O�� (2��)
CuSO4+SO2��+2H2O�� (2��)
ǿ�������� (6) (��1��=2��)
(3) 2SO2+O2+2H2O===2H2SO4 (2��)
��ij����ͨ��ʢƷ����Һ���Թ�����Ʒ����ɫ���ټ������ֳ��ֺ�ɫ��֤����SO2�� (3��)
(4)�� C (2��)
������ҺΪ��ɫ������Һ��ֻ��Na+������Һ����ɫ��һ����Fe2+��������ɫ��Ӧ����֤��Na+�Ĵ��ڡ�(����������Ҳ����) (3��)
(1) (1)(2)(5) (2��)
(2)2H2SO4(Ũ)+Cu
 CuSO4+SO2��+2H2O�� (2��)
CuSO4+SO2��+2H2O�� (2��)ǿ�������� (6) (��1��=2��)
(3) 2SO2+O2+2H2O===2H2SO4 (2��)
��ij����ͨ��ʢƷ����Һ���Թ�����Ʒ����ɫ���ټ������ֳ��ֺ�ɫ��֤����SO2�� (3��)
(4)�� C (2��)
������ҺΪ��ɫ������Һ��ֻ��Na+������Һ����ɫ��һ����Fe2+��������ɫ��Ӧ����֤��Na+�Ĵ��ڡ�(����������Ҳ����) (3��)
��

��ϰ��ϵ�д�
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
�����Ŀ

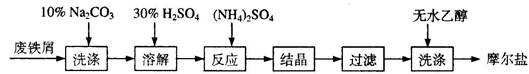
 6CaSiO3��P4O10 10C��P4O10
6CaSiO3��P4O10 10C��P4O10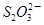 ��I2
��I2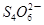 ��2I����һ�������ijά����C��Һ�м���a mol��L��1 I2��ҺV1mL����ַ�Ӧ����Na2S2O3��Һ�ζ�ʣ���I2, ����b mol��L��1Na2S2O3��ҺV2mL������Һ��ά����C�����ʵ�����___________mol��
��2I����һ�������ijά����C��Һ�м���a mol��L��1 I2��ҺV1mL����ַ�Ӧ����Na2S2O3��Һ�ζ�ʣ���I2, ����b mol��L��1Na2S2O3��ҺV2mL������Һ��ά����C�����ʵ�����___________mol��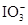 ��5
��5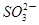 ��2H��
��2H��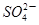 ��H2O���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�ijͬѧ���ʵ�����±���ʾ��
��H2O���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�ijͬѧ���ʵ�����±���ʾ�� 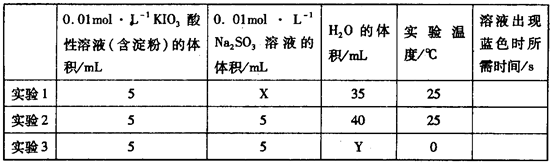
 Ce3����
Ce3���� ��Ӧ�������� ��Ӧ��
��Ӧ�������� ��Ӧ��