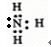��Ŀ����
X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺
��1������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� ����Ԫ�ط��ű�ʾ����
��2��X��Z�γɵ�3��1�Ļ�����A������Z������������Ӧ��ˮ����B��Ӧ�Ļ�ѧ����ʽ ����ˮ��Һ�� �ԣ������ӷ���ʽ����ԭ�� ��
��3����������������Ԫ�أ���Lͬһ���壬������������Ӧ��ˮ���ﻯѧʽΪ ����Ԫ�ع��嵥����H2��Ӧ����0.5mol��̬�⻯��ʱ������14.87kJ����������д��1mol���������������ϵ��Ȼ�ѧ����ʽ�� ��
��4����M������������ʯī��������NaHCO3��Һ�����Һ���е�⣬����������R��R���ȷֽ����ɻ�����Q��д����������R�ĵ缫��Ӧʽ�� ����R����Q�Ļ�ѧ����ʽ�� ��
��1������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����
��2��X��Z�γɵ�3��1�Ļ�����A������Z������������Ӧ��ˮ����B��Ӧ�Ļ�ѧ����ʽ
��3����������������Ԫ�أ���Lͬһ���壬������������Ӧ��ˮ���ﻯѧʽΪ
��4����M������������ʯī��������NaHCO3��Һ�����Һ���е�⣬����������R��R���ȷֽ����ɻ�����Q��д����������R�ĵ缫��Ӧʽ��
������M�ǵؿ��к�����ߵĽ���Ԫ�أ�ӦΪAl��X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ���C��N��O��H��P��Ԫ�أ�����X��Y��Z��L��M����Ԫ�ص�ԭ����������������X��Y��Z��L�ֱ�ΪH��C��N��OԪ�أ����Ԫ�������ɵĵݱ�����ж�Ԫ�ض�Ӧ���ʡ�������Ľṹ�����ʣ�
����⣺M�ǵؿ��к�����ߵĽ���Ԫ�أ�ӦΪAl��X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ���C��N��O��H��P��Ԫ�أ�����X��Y��Z��L��M����Ԫ�ص�ԭ����������������X��Y��Z��L�ֱ�ΪH��C��N��OԪ�أ�
��1��H��ԭ�Ӱ뾶��С��C��N��Oλ��ͬһ���ڣ�ԭ�Ӱ뾶��С˳��ΪC��N��O��ͬ�������϶���ԭ�Ӱ뾶������Al��ԭ�Ӱ뾶�������Al��C��N��O��H��
�ʴ�Ϊ��Al��C��N��O��H��
��2��H��N��Ԫ�ذ�ԭ����Ŀ��l��3�γɵĻ�����AΪNH3��Z������������Ӧ��ˮ����BΪHNO3�����߷�Ӧ����ʽΪ��NH3+HNO3�TNH4NO3���������Һ��笠�����ˮ��NH4++H2O?NH3?H2O+H+���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ��NH3+HNO3�TNH4NO3��NH4++H2O?NH3?H2O+H+��
��3������Se��������������Ԫ�أ���Oͬһ���壬������������Ӧ��ˮ���ﻯѧʽΪH2SeO4����Ԫ�ع��嵥����H2��Ӧ����0.5mol��̬�⻯��ʱ������14.87kJ��������1mol�������������������յ�����Ϊ14.87kJ��
=29.74kJ����Ӧ�Ȼ�ѧ����ʽΪ��Se��s��+H2��g��=H2Se��g����H=+29.74KJ/mol��
�ʴ𰸣�H2SeO4�� Se��s��+H2��g��=H2Se��g����H=+29.74KJ/mol��
��5����Al������������ʯī��������NaHCO3��Һ�����Һ���е�⣬����������R��Ϊ���������������������ȷֽ����ɻ�����QΪ��������������������������ͬʱ���ɶ�����̼�������缫��ӦʽΪ��Al-3e-+3HCO3-�TAl��OH��3��+3CO2������R����Q�Ļ�ѧ����ʽΪ��2Al��OH��3
Al2O3+3H2O��
�ʴ�Ϊ��Al-3e-+3HCO3-�TAl��OH��3��+3CO2����2Al��OH��3
Al2O3+3H2O��
��1��H��ԭ�Ӱ뾶��С��C��N��Oλ��ͬһ���ڣ�ԭ�Ӱ뾶��С˳��ΪC��N��O��ͬ�������϶���ԭ�Ӱ뾶������Al��ԭ�Ӱ뾶�������Al��C��N��O��H��
�ʴ�Ϊ��Al��C��N��O��H��
��2��H��N��Ԫ�ذ�ԭ����Ŀ��l��3�γɵĻ�����AΪNH3��Z������������Ӧ��ˮ����BΪHNO3�����߷�Ӧ����ʽΪ��NH3+HNO3�TNH4NO3���������Һ��笠�����ˮ��NH4++H2O?NH3?H2O+H+���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ��NH3+HNO3�TNH4NO3��NH4++H2O?NH3?H2O+H+��
��3������Se��������������Ԫ�أ���Oͬһ���壬������������Ӧ��ˮ���ﻯѧʽΪH2SeO4����Ԫ�ع��嵥����H2��Ӧ����0.5mol��̬�⻯��ʱ������14.87kJ��������1mol�������������������յ�����Ϊ14.87kJ��
| 1mol |
| 0.5mol |
�ʴ𰸣�H2SeO4�� Se��s��+H2��g��=H2Se��g����H=+29.74KJ/mol��
��5����Al������������ʯī��������NaHCO3��Һ�����Һ���е�⣬����������R��Ϊ���������������������ȷֽ����ɻ�����QΪ��������������������������ͬʱ���ɶ�����̼�������缫��ӦʽΪ��Al-3e-+3HCO3-�TAl��OH��3��+3CO2������R����Q�Ļ�ѧ����ʽΪ��2Al��OH��3
| ||
�ʴ�Ϊ��Al-3e-+3HCO3-�TAl��OH��3��+3CO2����2Al��OH��3
| ||
���������⿼���Ϊ�ۺϣ��漰Ԫ�ص��ƶϡ��뾶�Ƚϡ�����ˮ�⡢ԭ��ء����ӷ���ʽ�Լ��Ȼ�ѧ����ʽ���缫��Ӧʽ�Ȼ�ѧ����Ŀ��飬��Ŀ�Ѷ��еȣ�ע����ݵ����ʵ����Ԫ�ؽ��Ԫ�ص�ԭ��������ϵ�����ƶϣ�

��ϰ��ϵ�д�
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
�����Ŀ