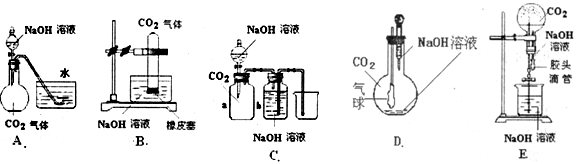
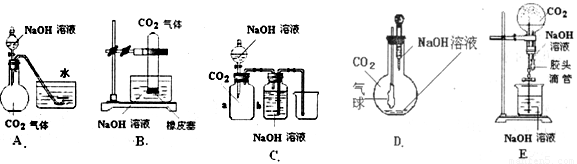
��Ŀ����
��10�֣� CO2��NaOH�ķ�Ӧ��һ���ܻ����Ļ�ѧ��Ӧ���кܶ��ʵ�鷽������ͨ���۲쵽����������˵��CO2��NaOH��Һ�����˷�Ӧ�����ṩ����ʵ����Ʒ������ƿ����ƿ����Һ©��������©�������ܡ���Ƥ�ܡ����ɼС��ձ�����Ͳ��ˮ��CO2���塢NaOH��Һ�Լ�����Ϊ�����õ���������ҩƷ��������λͬѧ�������ͼ��ʾ��A��E���װ�ã��Իش�
(1)��ͼA��������Һ©���е�NaOH��Һ������ƿʱ���������ˮ���е�ˮ�����뵽��ƿ�У���֤��CO2��NaOH��Һ�����˷�Ӧ����д���˹�����NaOH��Һ��CO2���ܷ����ķ�Ӧ���ӷ���ʽ��__________________��____________________��
(2)��ͼB��E����ָ���ܴﵽʵ��Ŀ�ĵ�װ��______________����B��C��D��E��գ���B�г���_______________________�����֤��CO2��NaOH�����˷�Ӧ��
(3)����״����һ�������CO2���建��ͨ��V L NaOH��Һ�У����CO2��NaOH����ʣ�࣮�ڷ�Ӧ�����Һ�м��������ij���ʯ��ˮ�õ�W g�����������������ܷ�ȷ��CO2����������ܣ����������������CO2������������ܣ�������ʲôʵ�飿��Ҫ˵��������ʵ���õ�������ʽ��ʾ��_________________________________________
(1) CO2 + OH��=HCO3�� HCO3��+OH����CO32��+ H2O
(2) A B D E�� ����������
(3) ![]()
����:��