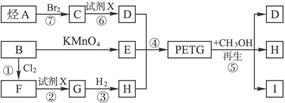
��Ŀ����
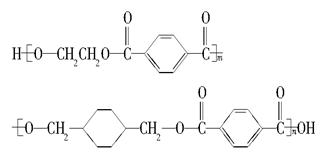
�ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ�PETG�²��Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ��PETG�Ľṹ��ʽ���£� ���ֲ��Ͽɲ�������ͼ��ʾ�ĺϳ�·��
���ֲ��Ͽɲ�������ͼ��ʾ�ĺϳ�·��

(1)![]()
(2)RCOOR1+R2OH![]() RCOOR2+R1OH(R��R1��R2��ʾ����)���÷�ӦΪȡ����Ӧ��������������⣺
RCOOR2+R1OH(R��R1��R2��ʾ����)���÷�ӦΪȡ����Ӧ��������������⣺
(1)�ߵķ�Ӧ������______________________________________________��
(2)д��I�Ľṹ��ʽ��__________________________________________��
(3)�ϳ�ʱӦ���Ƶĵ�������ʵ�����n(H)��n(E)��n(D)=___________��___________�� ___________ (��m��n��ʾ)��
(4)д����Ӧ�ڵĻ�ѧ����ʽ��______________________________��
(5)д��ͬʱ������������Ҫ���E������ͬ���칹��Ľṹ��ʽ��
�ٸ�ͬ���칹��ı��������ڵ�����̼ԭ���϶�����ȡ������
�ڸ�ͬ���칹����һ���������ܷ���������Ӧ��ˮ�ⷴӦ������FeCl3��Һ����ɫ��___________________________��________________________��________________________��
(1)ȡ����Ӧ
(2)I��![]()
(3)n��(m+n)��m
(4)CH2Br��CH2Br+2H2O![]() CH2OH��CH2OH+2HBr
CH2OH��CH2OH+2HBr

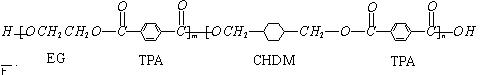
���������⿼����Ϣ�Ľ���������֪ʶ���ν��������������֪PETG�Ǿۺ���ɽṹ��ʽ��֪����ȡ���Ʒ������־ۺ���ĵ���ֱ��ǣ�DΪ�Ҷ���HOCH2��CH2OH����ô��BΪCH2=CH2��A��C�����ӳɷ�Ӧ��CΪCH2Cl��CH2Cl�������Ϣ���Ƴ�EΪ![]() ����ôBΪ
����ôBΪ![]() ��B��F����ȡ����Ӧ����ôFΪ
��B��F����ȡ����Ӧ����ôFΪ![]() ��F��G����ˮ�ⷴӦ��GΪ
��F��G����ˮ�ⷴӦ��GΪ![]() ��G��H�����ӳɷ�Ӧ������HΪ��
��G��H�����ӳɷ�Ӧ������HΪ��![]() ��PETG+CH3OH�����ķ�Ӧ��������Ϣ(2)��֪��Ӧ����ȡ����Ӧ��D��H��֪������I��
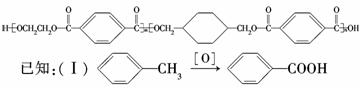
��PETG+CH3OH�����ķ�Ӧ��������Ϣ(2)��֪��Ӧ����ȡ����Ӧ��D��H��֪������I��![]() ,�ɾۺ����֪���ϳ�ʱӦ���Ƶĵ�������ʵ�����n(H)��n(E)��n(D)=n��(m+n)��m��E��ͬ���칹����һ���������ܷ���������Ӧ��ˮ�ⷴӦ������FeCl3��Һ����ɫ�����Ա��������ӵĹ������С�OH����ԭ�Ӹ����غ��֪������һ����CHO��һ��
,�ɾۺ����֪���ϳ�ʱӦ���Ƶĵ�������ʵ�����n(H)��n(E)��n(D)=n��(m+n)��m��E��ͬ���칹����һ���������ܷ���������Ӧ��ˮ�ⷴӦ������FeCl3��Һ����ɫ�����Ա��������ӵĹ������С�OH����ԭ�Ӹ����غ��֪������һ����CHO��һ��![]() �����Խṹ��ʽΪ
�����Խṹ��ʽΪ ��
��

�ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ�PETG�²�
�Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ��PETG�Ľṹ��ʽΪ��
 |
(��)RCOOR�䣫R��OH�D��RCOOR�士R��OH(R��R��R���ʾ����)
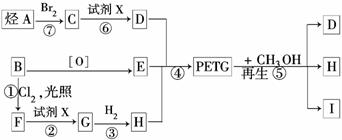 ���ֲ��Ͽɲ�������·�ߺϳɣ�
���ֲ��Ͽɲ�������·�ߺϳɣ�
�Իش��������⣺
(1)��Ӧ�ڢ�����Լ�X��________����Ӧ�١���������ȡ����Ӧ����________��
(2)д������I�Ľṹ��ʽ��____________________________________________.
(3)д����Ӧ�Ļ�ѧ����ʽ��___________________________________________
______________________________________________________________________.
(4)�ϳ�PETGʱ����������ʵ����ı�����ϵ�ǣ�
n(D)��n(E)��n(H)��________(��m��n��ʾ)��








 RCOOR2+R1OH��R��R1��R2����������
RCOOR2+R1OH��R��R1��R2����������