��Ŀ����
ʵ������ͼ��ʾװ���Ʊ���������������������ʵ�飬��ش��������⣺
��1��A�м���MnO2��B��ʢ��Ũ���ᣬ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
��2��C��ʢ����ɫʯ����Һ����C�е�������_______________������������Ľ�����____________________________________��
��3��D����NaBr��Һ��������������____________����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��4��E��ʢ��KI�͵��۵Ļ����Һ��������_______________________��
��5��F����AgNO3��Һ��������____________________����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��6��G��һ��Ӧʢ��________________����������_______________________����Ӧ�Ļ�ѧ����ʽΪ______________________��

������
��1��MnO2+4HCl(Ũ) ��2���ȱ�죬����ɫ������C�е��������Ȼ��⡢�������Ȼ�������ˮ�����ԣ�ʹʯ���죬��������Ư���ԣ�ʹ��Һ��ɫ�� ��3����Һ��ƣ�2NaBr+Cl2=2NaCl+Br2 ��4����Һ���� ��5���а�ɫ����������AgNO3+Cl2+H2O=AgCl+HclO+HCl ��6��NaOH��Һ���ն���Cl2����ֹ��Ⱦ������2NaOH+Cl2=NaCl+NaClO+H2O
|

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�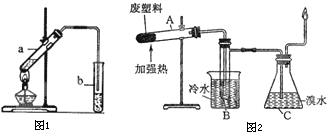
| ʵ�鲽�� | ʵ������ |
| I������Ϊ�٢ڢ۵�3֧�Թ��У��ֱ����1mL 20%��������Һ�����Թܢں͢��м���0.5mLϡ���ᣬ������3֧�Թ�ͬʱˮԡ����Լ5min | ������������ |
| II��ȡ�Թܢٺ͢ڣ���������������ͭ����Һ������������ | ������������ |
| III��ȡ�Թܢۣ��ȼ���NaOH��Һ����ҺpH�����ԣ��ټ�������������ͭ����Һ������������ | |
| ���ۣ�֤��������ϡ���������·�����ˮ�ⷴӦ | |
���Թ�a���������������Ļ�ѧ��Ӧ����ʽ��
���Թ�b��ʢ�ŵ��Լ���
����Ҫ��b���Ƶõ����������ӻ�����з��������Ӧ���õ�ʵ�������
���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ��������������Ӧ�Ѵﵽ��ѧƽ��״̬���У�����ţ�
a��λʱ�������1mol����������ͬʱ����1molˮ
b��λʱ�������1mol����������ͬʱ����1mol����
c��λʱ�������1mol�Ҵ���ͬʱ����1mol����
d����Ӧ���������淴Ӧ���������
e������и����ʵ�Ũ�Ȳ��ٱ仯
��3�����Ⱦ۱�ϩ���ϵõ��IJ��������
| ���� | ���� | ���� | ��ϩ | ��ϩ | �� | �ױ� | ̼ |
| ����������%�� | 12 | 24 | 12 | 16 | 20 | 10 | 6 |
���Թ�A�е����ղ�����Ϊ
���Թ�B�ռ����IJ�Ʒ�У�����ʹ���Ը��������Һ��ɫ�����ʣ������ʵ�һ�ȴ�����
����ƿC�й۲쵽��������

 ��2����ͼ��ʾװ��Ҳ������ȡNH3����Բ����ƿ�еĹ������ѡ��
��2����ͼ��ʾװ��Ҳ������ȡNH3����Բ����ƿ�еĹ������ѡ��