��Ŀ����
ij������ӣ���к���һ���ǻ��ᣨ��M��ʾ����M��̼���ṹ��֧��������ʽΪ
C4H6O5��1.34gM��������̼��������Һ��Ӧ�����ɱ�״���µ�����0.448L��M��һ�������¿ɷ�������ת����M A
A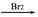 B
B C��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ���� �� ��
C��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ���� �� ��
A��M�Ľṹ��ʽΪHOOC��CHOH��CH2��COOH
B��B�ķ���ʽΪC4H4O4Br2
C����M�Ĺ��������ࡢ������ȫ��ͬ��ͬ���칹�廹��2��
D��C���ʲ���������ˮ
D
�������������1.34gA�����ʵ���Ϊ0.01mol��M��������̼��������Һ��Ӧ�����ɱ�״���µ�����0.448L��0.02mol�����Ի���Ȼ�����ĿΪ2��ȷ���л���M�Ľṹ��ʽΪHOOC��CHOH��CH2��COOH����A��ȷ����M�Ĺ��������ࡢ������ȫ��ͬ��ͬ���칹�廹��2�֣���B��ȷ��HOOC��CHOH��CH2��COOH�ڴ������ȵ������·�����ȥ��Ӧ���ɺ���˫��������A��AΪHOOCCH=CHCOOH����A���巢���˼ӳɷ�Ӧ��������B������B�ķ���ʽΪC4H4O4Br2������B��ȷ��HOOCCH=CHCOOH����������NaOH��Һ�����ӳɷ�Ӧ��C,CΪHOOCHOHCHOHCOOH����HOOCHOHCHOHCOOH��������ˮ����D����Ϊ����Ĵ𰸡�
���㣺�л���Ӧ������ʽ��ȷ�����л��������
���������⿼�����л���Ӧ������ʽ��ȷ�����л�������ʣ������ۺ��Խϴ���Ϣ�϶࣬�����ѶȽϴ�

 A
A B
B C��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ���� �� ��
C��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ���� �� �� ��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ����
�� ��
��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ����
�� ��