��Ŀ����
��1�����з����п���֤��2HI(g)�ٵ�λʱ��������n mol H2��ͬʱ����n mol HI
��һ��H��H�����ѵ�ͬʱ������H��I������
�۰ٷ����w(HI)=w(I2)
�ܷ�Ӧ����v(H2)=v(I2)=v(HI)/2ʱ
��c(HI)��c(H2)��c(I2)=2��1��1ʱ
���¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯
���¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯
������һ������������ƽ����Է����������ٱ仯
���¶Ⱥ����һ��ʱ������������ɫ���ٱ仯
���¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯
��2���������ޡ����˵������˵��2NO2![]() N2O4�ﵽƽ��״̬����_________________��
N2O4�ﵽƽ��״̬����_________________��
(1)�ڢޢ� (2)�ޢߢ���
�������۲�ڣ�1���ⷴӦ��������֪���˷�Ӧ�ڷ�Ӧǰ�����������������仯�����ڷ�Ӧ���κ�һ���Σ�������ѹǿ�������ı䡣��������������䡢�ܵ����ʵ������䣬��˻��������ܶȡ�ƽ����Է����������������ı䡣
�۲�ڣ�2���ⷴӦ����������Ӧǰ��������������ı䣬�����ƽ����Է�����������������ܶȶ������ı䣬����Ӧ����ƽ��״̬ʱ����Щ�����ٸı䣬��ʱc(NO2)�㶨����ɫ���ٱ仯��

��ϰ��ϵ�д�
�����Ŀ
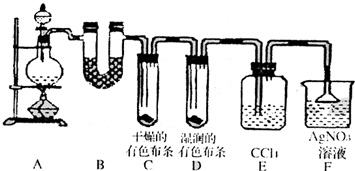 ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⣮
ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⣮ H2��g��+I2��g���Ѵﵽƽ�����( )
H2��g��+I2��g���Ѵﵽƽ�����( )