��Ŀ����
����Ŀ��A��B��C����Ԫ�����ڱ��еĶ�����Ԫ�أ����ǵĺ˵������������2����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2����Bԭ�ӵ������p����ĵ���Ϊ�����ṹ��C�ǵؿ��к�������Ԫ�ء�D�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ����������ö�Ӧ��Ԫ�ط��Ż�ѧʽ��գ�

��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��A������������Ӧ��ˮ���������������ԭ�Ӳ�ȡ �ӻ���
��3����A��B�γɵ������ӣ�AB������Ϊ�ȵ�����ķ����� ��
��4����̬Dԭ�ӵĺ�������Ų�ʽΪ ����ͼ�ǽ���Ca��D���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��D��ԭ�Ӹ�����Ϊ ��
��5����D�ĸ�̬��������Һ����εμ�B���⻯��ˮ��Һ���������ȳ�����ɫ����������ܽ��γ�����ɫ����Һ��д������ɫ�����ܽ�����ӷ���ʽ�� ��
���𰸡�
��1��C��O��N
��2��sp2
��3��N2��CO
��4��[Ar]3d104s1
��5��1:5
��6��Cu(OH)2+4NH3=[Cu(NH3)4]2+ +2OH��
��������
���������A��B��C����Ԫ�����ڱ��еĶ�����Ԫ�أ����ǵĺ˵������������Bԭ�ӵ������p����ĵ���Ϊ�����ṹ�����������Ų�Ϊ2s22p3����BΪNԪ�أ���2����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2������ԭ�Ӻ�������Ų�Ϊ1s22s1��1s22s22p2����ϣ�3������NԪ���γ������ӣ�AN-������AΪCԪ�أ�C�ǵؿ��к�����ߵ�Ԫ�أ�����C��OԪ�أ�D�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ���������DԪ��ԭ�Ӹ���������ֱ�Ϊ2��8��18��1����29��CuԪ�أ�
��1��C��N��SԪ����ͬһ����Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������C��O��N��
�ʴ�Ϊ��C��O��N��
��2��H2CO3��Cԭ������������ȫ���ɼ���û�йµ��Ӷԣ���1��C=O˫����2��C-O�������ӻ������ĿΪ3����ȡsp2�ӻ���
�ʴ�Ϊ��sp2��
��3����CN-��Cԭ�Ӽ�1������ɻ���1��Nԭ�ӣ��ɵõĵȵ�����N2����Nԭ�Ӽ�1������ɻ���1��Oԭ�ӣ��ɵõĵȵ�����CO��
�ʴ�Ϊ��N2��CO��
��4��D��ԭ��������29��ΪCuԪ�أ���ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d104s1���ɾ����ṹ��֪��Caԭ�Ӵ��ڶ��㣬�����к���Caԭ����ĿΪ8��![]() =1��Cuԭ�Ӵ��ھ����ڲ������ϡ����ģ�������Cu��ĿΪ1+4��
=1��Cuԭ�Ӵ��ھ����ڲ������ϡ����ģ�������Cu��ĿΪ1+4��![]() +4��
+4��![]() =5���ʸúϽ���Ca��Cu��ԭ�Ӹ�����Ϊ1��5��
=5���ʸúϽ���Ca��Cu��ԭ�Ӹ�����Ϊ1��5��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��1��5��
��5������ˮ�μӵ�����ͭ��Һ�У����ȷ�Ӧ����������ͭ�����������μӰ�ˮ��������ͭ�백ˮ��Ӧ�����İ���ͭ�����ӣ���Ӧ�����ӷ���ʽΪ��Cu(OH)2+4NH3=[Cu(NH3)4]2+ +2OH����
�ʴ�Ϊ��Cu(OH)2+4NH3=[Cu(NH3)4]2+ +2OH����

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�����Ŀ�������е�Ԫ�����в������Ƚ�����Ƶ���
A�����ʳ��ˮ���ռ� |
B���ϳɰ��еĴ��ϳ� |
C�����������еĴ����� |
D������еİ���ˮ̼�ữ |
����ˮɹ�ε�±ˮ�л����Ȼ�þ����±ˮΪԭ������þ��һ�й�����������ͼ��ʾ��
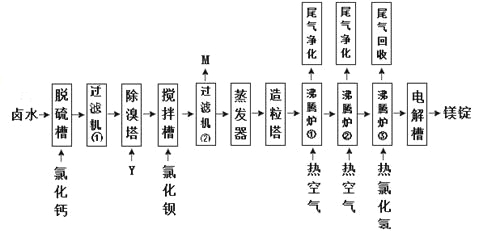
�ش��������⣺
��1������ۡ�����۾������ѳ�±ˮ�еģ������ӷ��ţ���M����Ҫ�ɷ��ǣ��ѧʽ����
��2������������Ҫ�����ӷ���ʽΪ��
��3������¯����������Ҫ�����ǡ�����¯��ͨ�����Ȼ������ҪĿ���ǡ�
��4�������������ĵ缫��Ӧ����ʽΪ��
��5����������������Ϊ���ò����ֱ�����ڱ����������еġ�



