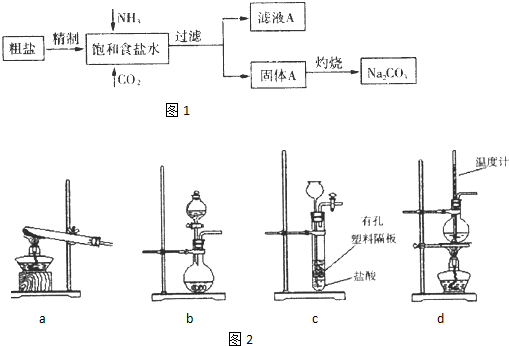
��Ŀ����
�ⶨNa2CO3�����нᾧˮ�ĺ���������Ҫ����ƽ�Ͻ���4�γ�����
��1����1����________�ij�������2����________�ij�������3����________�ij�������4����________�ij�����
��2����1��2�γ�����Ŀ����________����3��4�γ�����Ŀ����________��
��3��������������ѡ��ʵ����������������ĸ��д��________������Ҫ��������________��
A��������ƽ B���в� C���Թܼ����� D���ƾ��� E�������� F�������� G������ H�������� I��ʯ�������� J�����ż�
��4���������������NaHCO3���ʣ�����Ľᾧˮ������ƫ________��������ˮ�����û�з��ڸ������ڶ����ڿ�������ȴ��������Ľᾧˮ������ƫ________��
������
��1������������� ʢ��̼���ƾ���������� ������ȴ���Na2CO3�������� �ظ������εIJ��� ��2����̼���ƾ���������� ˵��̼���ƾ�����ȫʧȥ�ᾧˮ���õ���ˮ̼���Ƶ����� ��3��A��B��D��F��G��H��I�� ����ǯ�������ǡ�ҩ�� ��4���ͣ� ��
|
��ʾ��
�������������NaHCO3���ʣ��ڸ�������ʱ��ֽ⣬�����ڳ�ȥ�ᾧˮ���¶�ʱ����ֽ⣬�Ӷ�ʹ�ᾧˮ������ƫС���ᾧˮ�ĺ���Ҳƫ�ͣ����û�з��ڸ������ڶ����ڿ�������ȴ����Na2CO3�����տ����е�ˮ������ʹ��������Ľᾧˮ������ƫС���ᾧˮ�ĺ���Ҳƫ�͡�
|

 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�