��Ŀ����
A��B��C��D��F�dz����Ļ��������F�ڳ�������һ����ɫҺ�壬DΪǿ�ᣬ�������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺
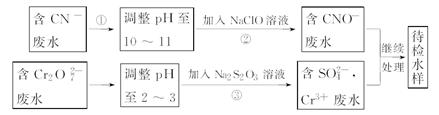
��1����A��B��C��D��Ϊ����Ԫ�صĻ����A��һ�ֳ����Ŀ�ʯ����Ҫ�ɷ֣���A��Ħ������Ϊ120 �� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
�� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
��2����A��B��C��D��Ϊ����Ԫ�صĻ������A��һ��������ֻ����10�����ӣ���
��A����ʽΪ__________��
�ڷ�Ӧ�ܵ����ӷ���ʽΪ________________________________________________
��ȡCu��Cu2O�Ļ������Ʒ12��0g�����뵽������D��ϡ��Һ�У�����ˮ���ռ����������壬��״���������Ϊ2��24L������Ʒ��Cu2O������Ϊ__________g��
��1��4FeS2+11O2 Fe2O3+8SO2����2�֣�
Fe2O3+8SO2����2�֣�
��2����NH3 ����2�֣�
��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O����2�֣�
��4��32g��2�֣�4��3 Ҳ�÷֣�
Ҳ�÷֣�
���������������1��A��һ�ֳ����Ŀ�ʯ����Ҫ�ɷ֣���A��Ħ������Ϊ120 ��A��FeS2,EΪ��������Ӧ�ٵĻ�ѧ����ʽΪ4FeS2+11O2
��A��FeS2,EΪ��������Ӧ�ٵĻ�ѧ����ʽΪ4FeS2+11O2 Fe2O3+8SO2��
Fe2O3+8SO2��
��2����A��B��C��D��Ϊ����Ԫ�صĻ����A��һ��������ֻ����10�����ӣ���A��NH3��
��E������,B��NO��C��NO2��F��ˮ��DΪ���ᣬ���Է�Ӧ�ܵ����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
����Cu��Cu2O�����ʵ����ֱ�Ϊx��y�����ݵ�ʧ�����غ㣬2��24L/22��4L/mol��3=2x+2y,���ߵ�����Ϊ12��0g��������64x+144y=12�����y=0��03mol,���Cu2O��������0��03mol��144g/mol=4��32g
���㣺�������Ļ�������ƶϼ����ʣ���ѧ����ʽ�����ӷ���ʽ����д��������ԭ��Ӧ�ļ���

 CoO2��LiC6����ŵ�ʱ����ص�������ӦʽΪ________________����ͼ��ʾ��װ�ù���ʱ���Ӻ����ӵ��ƶ�����ʱ�õ�ش���_________(��ŵ硱��硱)״̬��
CoO2��LiC6����ŵ�ʱ����ص�������ӦʽΪ________________����ͼ��ʾ��װ�ù���ʱ���Ӻ����ӵ��ƶ�����ʱ�õ�ش���_________(��ŵ硱��硱)״̬��

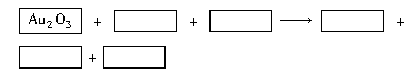
 Ag2O2����2KNO3��2K2SO4��2H2O
Ag2O2����2KNO3��2K2SO4��2H2O