��Ŀ����
��1 mol I2(g)��2 mol H2(g)����ij
(1)��ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ___________mol��L-1��
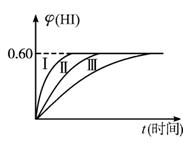
(2)���ı䷴Ӧ��������ij�����¦�(HI)�ı仯������(��)��ʾ���������������___________(�����������������)��
�ٺ��������£������¶� �ں��������£������¶� �ۺ��������£���С��Ӧ������� �ܺ��������£�����Ӧ������� �ݺ��¡����������£������ʵ�����
(3)�������¶Ȳ��䣬����һ��ͬ��
(1)0.05
(2)�ۢ�
(3)2b
������(1)���ƽ��ʱ����HI�����ʵ���Ϊn��
I2(g) + H2(g)![]() 2HI(g) ��H��0
2HI(g) ��H��0
��ʼ��1 mol 2 mol 0
�仯�� ![]()
![]() n
n
ƽ�⣺1-![]() 2
2![]() n
n
![]() ��100%=60%�����n=1.8 mol.
��100%=60%�����n=1.8 mol.
����ƽ��ʱc(I2)= =0.05 mol��L-1.
=0.05 mol��L-1.
(2)����ͼ��Ӧ���ʼӿ죬ƽ��״̬���ı䣬�ۢݷ�������.
(3)���顰��Чƽ�⡱����.���ݷ�Ӧ�ص㣬�����㡰���ϱ���ȡ�����![]() =
=![]() ������2b
������2b



 2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ
2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ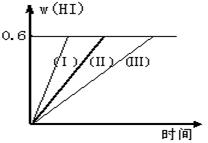
 2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��
2HI(g)����H��0������ƽ�⡣HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��