��Ŀ����
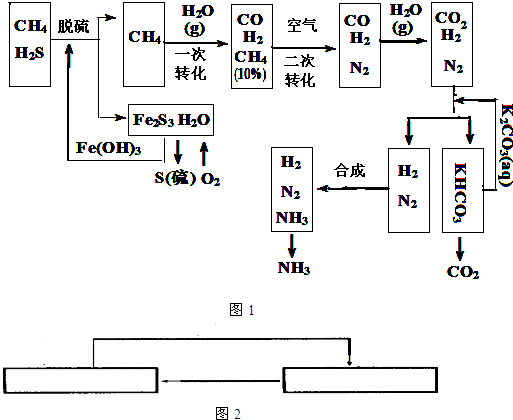
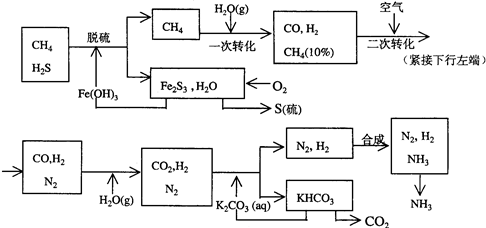
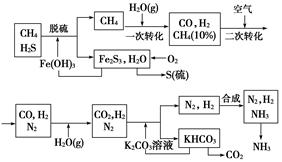
��12�֣�������Ȼ���ϳɰ��Ĺ�������ʾ�����£�
![]()

�����������̣����������գ�
��1����Ȼ������ʱ�Ļ�ѧ����ʽ��_______________________��
��2��K2CO3��aq����CO2��Ӧ�ڼ�ѹ�½��У���ѹ������������____________����ѡ�۷֣���
��a����������ԭ�� ��b����ɳ����ԭ�� ��c������к�ԭ��
��3����KHCO3�ֽ�õ���CO2��������_______________________��д��CO2��һ����Ҫ��;����
��4����������������ѭ����һ��Fe��OH��3ѭ��������K2CO3��aq��ѭ����������ͼ�б����������ͼ������ѭ����ѭ������ѭ�����ʣ���
![]()

![]() ��5����һ���¶Ⱥ�ѹǿ���ܱպϳɷ�Ӧ���У� H2��N2�������ƽ����Է�������Ϊ8.5�����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������ƽ��ʽ��Ϊ10��������ʱH2��ת���ʣ�д��������̣���
��5����һ���¶Ⱥ�ѹǿ���ܱպϳɷ�Ӧ���У� H2��N2�������ƽ����Է�������Ϊ8.5�����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������ƽ��ʽ��Ϊ10��������ʱH2��ת���ʣ�д��������̣���
��1��3H2S��2Fe��OH��3![]() Fe2S3+6H2O��2�֣�
Fe2S3+6H2O��2�֣�
��2��b��2�֣�
��3����������ۻ���������ȣ�����������Ҳ���֣ݣ�2�֣�
��4�� ��2�֣�
��2�֣�
��5���������������Ϊ1mol������Ϊx��������Ϊ��1-x����
���У� 28x+2(1-x)=8.5��ã�N2��x=0.25mol H2��1mol-0.25mol=0.75mol
����ƽ��ʱN2ת��y����
N2 + 3H2 ![]() 2NH3
2NH3
��ʼ 0.25mol 0.75mol 0
�仯 y 3y 2y
ƽ�� (0.25-y)mol (0.75-3y)mol 2ymol
���У�![]() ��ã�y=0.075mol
��ã�y=0.075mol
��������ת����Ϊ��![]() �����������ⷨ���ո��֣���4�֣�
�����������ⷨ���ո��֣���4�֣�