��Ŀ����
��10�֣���֪����25��ʱ������ʵ���ƽ������Ka��CH3COOH����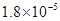 ��Ka��HSCN����0.13�����ܵ���ʵ��ܶȻ�������Kap��CaF2����
��Ka��HSCN����0.13�����ܵ���ʵ��ܶȻ�������Kap��CaF2����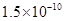
��25��ʱ��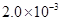 mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ��
mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ��
�����������Ϣ�ش��������⣺
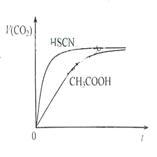
��1��25��ʱ����20mL 0.10 mol��L-1 CH3COOH��Һ��20mL 0.10 mol��L-1HSCN��Һ�ֱ���20mL 0.10 mol��L-1NaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���仯��ʾ��ͼΪͼ��ʾ����Ӧ��ʼ�Σ�������Һ����CO2��������ʴ������Բ����ԭ���� ����Ӧ��������������Һ�У�c��CH3COO-�� c��SCN-�����������������������
��2��25��ʱ��HF����ƽ�ⳣ������ֵKa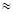 ����ʽ��˵���ó��ó��������� ��
����ʽ��˵���ó��ó��������� ��
��3��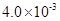 mol��L-1HF��Һ��
mol��L-1HF��Һ��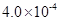 mol��L-1
CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯����ͨ����ʽ����˵���Ƿ��г���������
mol��L-1
CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯����ͨ����ʽ����˵���Ƿ��г���������
��1��HSCN�����Ա�CH3COOHǿ������Һ��������Ũ�Ƚϴ���������Һ��NaHCO3��Һ�ķ�Ӧ���ʽϿ죻��
��2��Ka��0.4��10-3
��3���������
��������
�����������1��HSCN�����Ա�CH3COOHǿ������Һ��������Ũ�Ƚϴ���������Һ��NaHCO3��Һ�ķ�Ӧ���ʽϿ죻��Ӧ����������ΪCH3COONa��NaSCN,����CH3COOH��������HSCN�����ԣ�����CH3COONaˮ��̶ȴ����c��CH3COO-����c��SCN-����
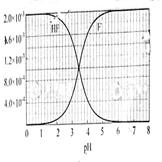
��2��HF����ƽ�ⳣ��Ka����c��H+����c��F-������ c��HF��,����c��H+���� c��F-���� c��HF�����ǵ���ﵽƽ��ʱ��Ũ�ȣ�ѡ���м��ͼ����⡣����ͼ��pH��4ʱ��c��H+����10-4�� c��F-����1.6��10-3��c��HF����4.0��10-4������Ka��0.4��10-3��
��3��pH��4.0����c��H+����10-4
��ʱ����HF�����֪������c��F-����1.6��10-3
����Һ�е�c��Ca2+����2.0��10-4
c2��F-����c��Ca2+����5.12��10-10
5.12��10-10����Kap��CaF2����1.5��10-10����˴�ʱ����������������
���㣺�������ƽ�ⳣ�����ܶȻ��������йؼ��㡢ͼ��ʶ���Լ�����ˮ���Ӧ�õ�
�����������ۺ���ǿ���ѶȽϴ���Ƚ�ȫ��ؿ����˵���ƽ�ⳣ�����ܽ�ƽ�ⳣ����֪ʶ���ݡ�Ҫ��ѧ�����ͼ��Ҫ����ƽ�ⳣ���ı���ʽ�ͺ��壺ƽ��ʱ����Һ�еĸ���������ӵ�Ũ�ȡ�Ҫ�����ܽ�ƽ��ĺ��壺�ﵽ������Һʱ�����ֵ�����������������С������������ȷƽ�ⳣ�������¶ȱ仯�ģ�������Һ�е�����Ũ�ȵ�ʵ��ֵ�������仯��

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�(14��)��֪����25��ʱ������ʵ���ƽ������Ka��CH3COOH���� ��Ka��HSCN����0.13�����ܵ���ʵ��ܶȻ�������Kap��CaF2����
��Ka��HSCN����0.13�����ܵ���ʵ��ܶȻ�������Kap��CaF2����
��25��ʱ�� mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ��
mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ��

�����������Ϣ�ش��������⣺
��1��25��ʱ��HF����ƽ�ⳣ������ֵKa= ��
��2��25��ʱ����20mL 0.10 mol��L-1 CH3COOH��Һ��20mL 0.10 mol��L-1HSCN��Һ�ֱ���20mL 0.10 mol��L-1NaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���仯��ʾ��ͼΪͼ2��ʾ��
��Ӧ��ʼ�Σ�������Һ����CO2��������ʴ������Բ����ԭ���� ����Ӧ��������������Һ�У�c��CH3COO-�� c��SCN-�����������������������
��3�� mol��L-1HF��Һ��
mol��L-1HF��Һ�� mol��L-1 CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯���� ����С����ޡ�������������
mol��L-1 CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯���� ����С����ޡ�������������
��4����֪CH3COONH4��ҺΪ���ԣ���֪CH3COOH��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�NH4HCO3��Һ��pH 7�����������������������
��ͬ�¶��µ�Ũ�ȵ���������Һ��?
| A��NH4Cl | B��NH4SCN? | C��CH3COONH4 | D��NH4HCO3 |
��pH�ɴ�С��˳�������ǣ� ������ţ�
 ��2010?�㽭����֪��
��2010?�㽭����֪��



 ��Ka��HSCN����0.13�����ܵ���ʵ��ܶȻ�������Kap��CaF2����
��Ka��HSCN����0.13�����ܵ���ʵ��ܶȻ�������Kap��CaF2����
 mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ��
mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ��

 mol��L-1HF��Һ��
mol��L-1HF��Һ�� mol��L-1
CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯���� ����С����ޡ�������������
mol��L-1
CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯���� ����С����ޡ�������������