��Ŀ����
����ʯ����Ҫ�ɷ���K2SO4?Al2��SO4��3?2Al2O3?6H2O������������Fe2O3���ʣ���������ʯ�Ʊ������������������£�

��1������¯�з�����Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ����������______��������1molAl2O3����ת�Ƶĵ�����Ϊ______��
���÷�Ӧ����������______��������1molAl2O3����ת�Ƶĵ�����Ϊ______��
��2���������1.12L¯��ͨ��100mL0.5mol/L NaOH��Һ�У��õ�һ��������Һ�������Һ�и�������Ũ���ɴ�С������˳��Ϊ______��
��3�������ܽ�ʱ��Ӧ�����ӷ���ʽΪ______��
��4����������к���Fe2O3������Լ���______��
��5��ĸҺ��������Ҫ�ɷֵĻ�ѧʽΪ______����Һ����pH���ˡ�ϴ�ӿɵ�Al��OH��3������֤��������ϴ�Ӹɾ���ʵ�������������______��
�⣺��1����Ӧ����Ԫ�ػ��ϼ���Al2��SO4��3��+6�۽���ΪSO2��+4�ۣ���Al2��SO4��3������������Ӧ����������Ԫ�ػ��ϼ���0������ΪSO2��+4�ۣ�����Ϊ��ԭ��������1molAl2O3��Ҫ������ʵ���Ϊ1mol�� =1.5mol��ת�Ƶ��ӵ����ʵ���Ϊ1.5mol��4=6mol��ת�Ƶ�����ĿΪ6mol��6.02��1023mol-1=3.612��1024��
=1.5mol��ת�Ƶ��ӵ����ʵ���Ϊ1.5mol��4=6mol��ת�Ƶ�����ĿΪ6mol��6.02��1023mol-1=3.612��1024��
�ʴ�Ϊ��Al2��SO4��3��3.612��1024��
��2��1.12L������������ʵ���Ϊ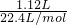 =0.05mol��n��NaOH��=0.1L��0.5mol/L=0.05mol��n��SO2����n��NaOH��=0.05mol��0.05mol=1��1���ʷ�Ӧ����Һ������ΪNaHSO3����Һ�����ԣ�˵��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�ͬʱˮ�������������ӣ���c��H+����c��SO32-����HSO3-����̶Ȳ���c��HSO3-����c��H+������Һc��OH-������ˮ�������ɣ�Ũ�Ⱥ�С������Һ������Ũ���ɴ�С������˳��Ϊc��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
=0.05mol��n��NaOH��=0.1L��0.5mol/L=0.05mol��n��SO2����n��NaOH��=0.05mol��0.05mol=1��1���ʷ�Ӧ����Һ������ΪNaHSO3����Һ�����ԣ�˵��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�ͬʱˮ�������������ӣ���c��H+����c��SO32-����HSO3-����̶Ȳ���c��HSO3-����c��H+������Һc��OH-������ˮ�������ɣ�Ũ�Ⱥ�С������Һ������Ũ���ɴ�С������˳��Ϊc��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��3���ɹ������̿�֪�������ܽ�Ϊ������������������Һ��Ӧ����ƫ�����ƣ����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��4�������м������ᣬ�μ�KSCN��Һ������Һ����ɫ��˵�������к���Fe2O3��
�ʴ�Ϊ�����ᡢKSCN��Һ��
��5���ɹ������̿�֪�������ܽ����õ���Һ�к���K+��Na+��SO42-��AlO2-��OH-�ȣ��������PHֵ��AlO2-ת��ΪAl��OH��3��ĸҺ��������Ҫ��K+��Na+��SO42-����������ΪK2SO4��Na2SO4��
Al��OH��3�����ḽ��K+��Na+��SO42-�ȣ�ȡ����ϴ��Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ���
�ʴ�Ϊ��K2SO4��Na2SO4��ȡ����ϴ��Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ���
��������1������Ԫ�ػ��ϼ۽��͵ķ�Ӧ������������
��Ӧ����������Ԫ�ػ��ϼ���0������ΪSO2��+4�ۣ�����Ϊ��ԭ������������1molAl2O3��Ҫ������ʵ�����ת�Ƶ��������ʵ�4�����ٸ���N=nNAת�Ƶ�����Ŀ��
��2������n=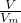 ���������������ʵ���������n��SO2����n��NaOH��ȷ����Ӧ����ٽ����Һ�����Խ����жϣ�
���������������ʵ���������n��SO2����n��NaOH��ȷ����Ӧ����ٽ����Һ�����Խ����жϣ�
��3���ɹ������̿�֪�������ܽ�Ϊ������������������Һ��Ӧ����ƫ�����ƣ�
��4�������м������ᣬ�μ�KSCN��Һ������Һ����ɫ��˵�������к���Fe2O3��
��5���ɹ������̿�֪�������ܽ����õ���Һ�к���K+��Na+��SO42-��AlO2-��OH-�ȣ��������PHֵ��AlO2-ת��ΪAl��OH��3��ĸҺ��������Ҫ��K+��Na+��SO42-��Al��OH��3�����ḽ��
K+��Na+��SO42-�ȣ���BaCl2��Һ��������ϴ��Һ���Ƿ���SO42-��ȷ���Ƿ�ϴ�Ӹɾ���
���������⿼��ѧ���Թ������̵����⡢������ԭ��Ӧ������Ũ�ȴ�С�Ƚϡ����Ӽ��顢ʵ�鷽����Ƶȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ��������������������Ŀ�Ѷ��еȣ���2����ע���ȸ��ݼ���ȷ����Ӧ����Һ�����ʣ�
 =1.5mol��ת�Ƶ��ӵ����ʵ���Ϊ1.5mol��4=6mol��ת�Ƶ�����ĿΪ6mol��6.02��1023mol-1=3.612��1024��
=1.5mol��ת�Ƶ��ӵ����ʵ���Ϊ1.5mol��4=6mol��ת�Ƶ�����ĿΪ6mol��6.02��1023mol-1=3.612��1024���ʴ�Ϊ��Al2��SO4��3��3.612��1024��
��2��1.12L������������ʵ���Ϊ
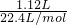 =0.05mol��n��NaOH��=0.1L��0.5mol/L=0.05mol��n��SO2����n��NaOH��=0.05mol��0.05mol=1��1���ʷ�Ӧ����Һ������ΪNaHSO3����Һ�����ԣ�˵��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�ͬʱˮ�������������ӣ���c��H+����c��SO32-����HSO3-����̶Ȳ���c��HSO3-����c��H+������Һc��OH-������ˮ�������ɣ�Ũ�Ⱥ�С������Һ������Ũ���ɴ�С������˳��Ϊc��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
=0.05mol��n��NaOH��=0.1L��0.5mol/L=0.05mol��n��SO2����n��NaOH��=0.05mol��0.05mol=1��1���ʷ�Ӧ����Һ������ΪNaHSO3����Һ�����ԣ�˵��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�ͬʱˮ�������������ӣ���c��H+����c��SO32-����HSO3-����̶Ȳ���c��HSO3-����c��H+������Һc��OH-������ˮ�������ɣ�Ũ�Ⱥ�С������Һ������Ũ���ɴ�С������˳��Ϊc��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-�����ʴ�Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��3���ɹ������̿�֪�������ܽ�Ϊ������������������Һ��Ӧ����ƫ�����ƣ����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��4�������м������ᣬ�μ�KSCN��Һ������Һ����ɫ��˵�������к���Fe2O3��
�ʴ�Ϊ�����ᡢKSCN��Һ��
��5���ɹ������̿�֪�������ܽ����õ���Һ�к���K+��Na+��SO42-��AlO2-��OH-�ȣ��������PHֵ��AlO2-ת��ΪAl��OH��3��ĸҺ��������Ҫ��K+��Na+��SO42-����������ΪK2SO4��Na2SO4��
Al��OH��3�����ḽ��K+��Na+��SO42-�ȣ�ȡ����ϴ��Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ���
�ʴ�Ϊ��K2SO4��Na2SO4��ȡ����ϴ��Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ���
��������1������Ԫ�ػ��ϼ۽��͵ķ�Ӧ������������
��Ӧ����������Ԫ�ػ��ϼ���0������ΪSO2��+4�ۣ�����Ϊ��ԭ������������1molAl2O3��Ҫ������ʵ�����ת�Ƶ��������ʵ�4�����ٸ���N=nNAת�Ƶ�����Ŀ��
��2������n=
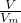 ���������������ʵ���������n��SO2����n��NaOH��ȷ����Ӧ����ٽ����Һ�����Խ����жϣ�
���������������ʵ���������n��SO2����n��NaOH��ȷ����Ӧ����ٽ����Һ�����Խ����жϣ���3���ɹ������̿�֪�������ܽ�Ϊ������������������Һ��Ӧ����ƫ�����ƣ�
��4�������м������ᣬ�μ�KSCN��Һ������Һ����ɫ��˵�������к���Fe2O3��
��5���ɹ������̿�֪�������ܽ����õ���Һ�к���K+��Na+��SO42-��AlO2-��OH-�ȣ��������PHֵ��AlO2-ת��ΪAl��OH��3��ĸҺ��������Ҫ��K+��Na+��SO42-��Al��OH��3�����ḽ��
K+��Na+��SO42-�ȣ���BaCl2��Һ��������ϴ��Һ���Ƿ���SO42-��ȷ���Ƿ�ϴ�Ӹɾ���
���������⿼��ѧ���Թ������̵����⡢������ԭ��Ӧ������Ũ�ȴ�С�Ƚϡ����Ӽ��顢ʵ�鷽����Ƶȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ��������������������Ŀ�Ѷ��еȣ���2����ע���ȸ��ݼ���ȷ����Ӧ����Һ�����ʣ�

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ