��Ŀ����
A��B��C��D��ԭ���������������ͬһ��ͬ��Ԫ�أ�A��B�ǽ���Ԫ�أ�C��D�Ƿǽ���Ԫ�ء�
(1)A��B���Ե�����������Ӧ��ˮ������Է�Ӧ�����κ�ˮ���÷�Ӧ�����ӷ���ʽΪ____________________��
(2)A��C ���γɻ�����A2C���û�����ĵ���ʽΪ______________��
(3)C�ĵͼ�������ͨ��D���ʵ�ˮ��Һ�У�������Ӧ�Ļ�ѧ����ʽΪ____________________________��
(4)A��B��C��D����Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳���ǣ������ӷ��ű�ʾ����_____��_____��______��______��
(5)����Ԫ���н�������ǿ��(��Ԫ�ط���)____________;�ǽ�������ǿ��_______________
(1)A��B���Ե�����������Ӧ��ˮ������Է�Ӧ�����κ�ˮ���÷�Ӧ�����ӷ���ʽΪ____________________��
(2)A��C ���γɻ�����A2C���û�����ĵ���ʽΪ______________��
(3)C�ĵͼ�������ͨ��D���ʵ�ˮ��Һ�У�������Ӧ�Ļ�ѧ����ʽΪ____________________________��
(4)A��B��C��D����Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳���ǣ������ӷ��ű�ʾ����_____��_____��______��______��
(5)����Ԫ���н�������ǿ��(��Ԫ�ط���)____________;�ǽ�������ǿ��_______________
(1)Al(OH)3+OH-��AlO2-+2H2O
(2)
(3)SO2+Cl2+2H2O��H2SO4+2HCl
(4)S2->Cl->Na+>Al3+
(5)Na��Cl
(2)

(3)SO2+Cl2+2H2O��H2SO4+2HCl
(4)S2->Cl->Na+>Al3+
(5)Na��Cl

��ϰ��ϵ�д�
 ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ
A��B��C��D��ԭ������������������ֶ�����Ԫ�أ��ס��ҡ������������������е����ֻ�����Ԫ����ɵĻ������������CԪ���γɵĵ��ʣ���֪����+��=��+������+��=��+����������0.1mol/L����Һ��pHΪ13��������˵����ȷ���ǣ�������
| A��Ԫ��C�γɵĵ��ʿ����ڵ�ȼ�����ֱ���Ԫ��A��B��D�γɵĵ��ʻ��ϣ����û���������ڹ��ۼ� | B��Ԫ��B��C��D��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��r��D����r��C����r��B�� | C��1.0 L 0.1 mol/L����Һ�к��������ܵ����ʵ���С��0.1 mol | D��1 mol��������������ȫ��Ӧ��ת��Լ1.204��1024������ |
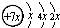 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺

 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ �����бȽ�����ȷ���ǣ�������
�����бȽ�����ȷ���ǣ�������