��Ŀ����
����ҹ�����������һ������ȼ�ϵ�أ�һ���缫ͨ���������һ���缫ͨ���������� ����C4H10�������ͣ�����صĵ�����Dz�����Y2O3��ZrO2���壬���ڸ������ܴ���O2-���ش��������⣺
��1��C4H10�������顢�춡�����ֽṹ��д���춡���һ��ȡ����Ľṹ��ʽ______��______��______��
��2�����ȼ�ϵ�طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��______��
��3�������ص����������ķ�Ӧ��O2+4e-=2O2-�����������ĵ缫��Ӧ����ʽ��______��
��4������������O2-��______���ƶ��������·�ͷŵ��ӵ���______����
��1��C4H10�������顢�춡�����ֽṹ��д���춡���һ��ȡ����Ľṹ��ʽ______��______��______��
��2�����ȼ�ϵ�طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��______��
��3�������ص����������ķ�Ӧ��O2+4e-=2O2-�����������ĵ缫��Ӧ����ʽ��______��
��4������������O2-��______���ƶ��������·�ͷŵ��ӵ���______����
��1���춡������ԭ�ӵ����������֣�������һ�ȴ��������֣���CH2Cl-CH��CH3��2����CH3��3Cl��CH3-CCl��CH3��2���ʴ�Ϊ��CH2Cl-CH��CH3��2����CH3��3Cl��CH3-CCl��CH3��2��
��2������ȼ�ϵ�طŵ�ʱ������Ӧ�Ƕ���ȼ�յĻ�ѧ����ʽ����2C4H10+13O2�T8CO2+10H2O���ʴ�Ϊ��2C4H10+13O2�T8CO2+10H2O��
��3������ȼ�ϵ�طŵ�ʱ������������ȼ�϶���ʧ���ӵ�������Ӧ���Ҹ�����Ӧ=�ܷ�Ӧ-������Ӧ������ӦΪ��
13O2+52e-=26O2-���ܷ�Ӧ�ǣ�2C4H10+13O2�T8CO2+10H2O�����Ը�����Ӧ�ǣ�2C4H10+26O2--52e-=8CO2+10H2O���ʴ�Ϊ��2C4H10+26O2--52e-=8CO2+10H2O��
��4����ȼ�ϵ���У����������������������������������������Ե������O2-���ƶ������������·�ͷŵ��ӣ��ʴ�Ϊ����������
��2������ȼ�ϵ�طŵ�ʱ������Ӧ�Ƕ���ȼ�յĻ�ѧ����ʽ����2C4H10+13O2�T8CO2+10H2O���ʴ�Ϊ��2C4H10+13O2�T8CO2+10H2O��
��3������ȼ�ϵ�طŵ�ʱ������������ȼ�϶���ʧ���ӵ�������Ӧ���Ҹ�����Ӧ=�ܷ�Ӧ-������Ӧ������ӦΪ��
13O2+52e-=26O2-���ܷ�Ӧ�ǣ�2C4H10+13O2�T8CO2+10H2O�����Ը�����Ӧ�ǣ�2C4H10+26O2--52e-=8CO2+10H2O���ʴ�Ϊ��2C4H10+26O2--52e-=8CO2+10H2O��
��4����ȼ�ϵ���У����������������������������������������Ե������O2-���ƶ������������·�ͷŵ��ӣ��ʴ�Ϊ����������

��ϰ��ϵ�д�
 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
�����Ŀ

 ��CH3CH2CH3 ��HC
��CH3CH2CH3 ��HC CCH3
CCH3  ��CH3CH��CHCH3
��CH3CH��CHCH3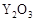 ��
��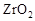 ���壬���ڸ������ܴ���
���壬���ڸ������ܴ��� ���ش��������⣺���Զ��飨
���ش��������⣺���Զ��飨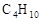 ���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��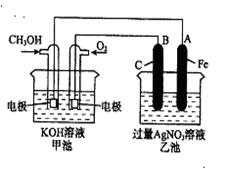
 ��CH3CH2CH3 ��HC
��CH3CH2CH3 ��HC CCH3
CCH3 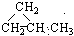 ��CH3CH��CHCH3
��CH3CH��CHCH3 ��
�� ���壬���ڸ������ܴ���
���壬���ڸ������ܴ���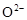 ���ش��������⣺���Զ��飨
���ش��������⣺���Զ��飨 ���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��