��Ŀ����
��14�֣����¶�t���£�ijBa(OH)2��ϡ��Һ��c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12�������Һ����μ���pH=b��NaHSO4����û����Һ�IJ���pH���±���ʾ��
��1�����������жϣ�t��___________25��(����ڡ�����С�ڡ����ڡ�)�����¶���ˮ�����ӻ�����Kw = ___________��
��2��b=____________��x =" ______mL" ��
��3����Ӧ�۵����ӷ���ʽΪ____________________________
��4�������¶��µ�Ba(OH)2��Һȡ��1mL����ˮϡ����1L����ϡ�ͺ���Һ��
c(Ba2+)�sc(OH��)= ��
��5�� ��NaHSO4��ͬ�� NaHSO3��NaHCO3ҲΪ��ʽ�Ρ���֪NaHSO3��Һ�����ԣ�NaHCO3��Һ�ʼ��ԡ�����Ũ�Ⱦ�Ϊ0.1mol/L��NaHSO3��Һ��NaHCO3��Һ����Һ�и����ӵ����ʵ���Ũ�ȴ������й�ϵ(R��ʾS��C)�����п�����ȷ����___________������ȷ�𰸵ı�ţ���
�� ��A��c( )��c(
)��c( )��c(
)��c( )��c(
)��c( )��c(
)��c( )
)
����B��c( )��c(
)��c( )��c(
)��c( )��2c(
)��2c( )��c(
)��c( )
)
����C��c( )��c(
)��c( )��c(
)��c( )��c(
)��c( )
)
����D������Һ��c( )��c(
)��c( )��c(
)��c( )�ֱ����
)�ֱ����
| ��� | �������������/mL | �������Ƶ����/mL | ��Һ��pH |
| �� | 33.00 | 0.00 | 8 |
| �� | 33.00 | x | 7 |
| �� | 33.00 | 33.00 | 6 |
��2��b=____________��x =" ______mL" ��
��3����Ӧ�۵����ӷ���ʽΪ____________________________
��4�������¶��µ�Ba(OH)2��Һȡ��1mL����ˮϡ����1L����ϡ�ͺ���Һ��
c(Ba2+)�sc(OH��)= ��
��5�� ��NaHSO4��ͬ�� NaHSO3��NaHCO3ҲΪ��ʽ�Ρ���֪NaHSO3��Һ�����ԣ�NaHCO3��Һ�ʼ��ԡ�����Ũ�Ⱦ�Ϊ0.1mol/L��NaHSO3��Һ��NaHCO3��Һ����Һ�и����ӵ����ʵ���Ũ�ȴ������й�ϵ(R��ʾS��C)�����п�����ȷ����___________������ȷ�𰸵ı�ţ���
�� ��A��c(
 )��c(
)��c( )��c(
)��c( )��c(
)��c( )��c(
)��c( )
)����B��c(
 )��c(
)��c( )��c(
)��c( )��2c(
)��2c( )��c(
)��c( )
)����C��c(
 )��c(
)��c( )��c(
)��c( )��c(
)��c( )
)����D������Һ��c(
 )��c(
)��c( )��c(
)��c( )�ֱ����
)�ֱ������ÿ��2�֣�
��1��> , 1��10��12
��2�� 4, 27
��3�� Ba2+ + 2OH�� + 2H+ + SO42�� = BaSO4 ��+ 2H2O
��4�� 1:20
��5�� ABC
��1��> , 1��10��12
��2�� 4, 27
��3�� Ba2+ + 2OH�� + 2H+ + SO42�� = BaSO4 ��+ 2H2O
��4�� 1:20
��5�� ABC
�����������1��t���£�ijBa(OH)2��ϡ��Һ��c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12����Kw = c(H+) ��c(OH-)=10-��a+b��=1��10-12>1��10-14������t>25��
��2��δ��������������Һ��Ba(OH)2��ϡ��ҺpH=8��˵��Ba(OH)2��ϡ��Һ��c(H+)=10-8 mol/L����a=8,b=4��33mL��Ba(OH)2��ϡ��Һ����xmL��pH=4��NaHSO4��pH=7��˵����ʱ��Һ�Լ��ԣ���c(OH-)��V(Ba(OH)2)- c(H+)��x=1��10-12/1��10-7��(x+33),���x=27��
��3����Ӧ���ǵ������Ba(OH)2��Һ��NaHSO4��Һ��Ӧ����Ϻ���Һ�����ԣ��������ӷ���ʽΪBa2+ + 2OH�� + 2H+ + SO42�� = BaSO4 ��+ 2H2O
��4��Ba(OH)2��ǿ�1mL��Ba(OH)2��Һ��c(Ba2+)="0.5" c(OH��)=1/2��10-4mol/L��ϡ��1000����c(Ba2+)=5��10-8mol/L��c(OH��)=1��10-7mol/L< c(H+)������Һ�в����ܴ���c(OH��) < c(H+)�����Դ�ʱϡ�ͺ����Һ������������Ũ�Ȱ���ˮ���㣬c(OH��)=1��10-6mol/L����c(Ba2+)�sc(OH��)= 5��10-8mol/L/1��10-6mol/L=1:20��
��5��NaHSO3��Һ�����ԣ�˵��HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ�NaHCO3��Һ�ʼ��ԣ�˵��HCO3-��ˮ��̶ȴ��������̶ȡ�A����R��SԪ�أ���c(Na+)��c(HRO3-)��c(H+)��c(RO32-)��c(OH-)����ȷ��B������R������Ԫ�أ������ϵ���غ���ɣ���ȷ��C������R������Ԫ�أ������������غ���ɣ�c(Na+)��c(HRO3-)��c(RO32-)��c(H2RO3)����c(Na+)��c(H+)��c(HRO3-)��2c(RO32-)��c(OH-)���ϣ�����
c(H+)��c(H2RO3)��c(RO32-)��c(OH-)����ȷ��D������Һ��c(Na+)��ȣ�NaHSO3��Һc(RO32-)�ϴ�c(HRO3-)���жϣ����ʰ���ѡABC��

��ϰ��ϵ�д�
�����Ŀ
 HCO3-+H+��ƽ�ⳣ��K1=______������֪��10-5.60=2.5��10-6��
HCO3-+H+��ƽ�ⳣ��K1=______������֪��10-5.60=2.5��10-6��
 CO2(g)+H2(g) ��H��0����850��ʱ��ƽ�ⳣ��K=1��
CO2(g)+H2(g) ��H��0����850��ʱ��ƽ�ⳣ��K=1��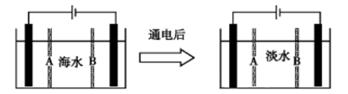
 ��+c��HCO
��+c��HCO ��]
��] CH3OH(g)��H2O(g)���������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ(ע��T1��T2������300 ��)��
CH3OH(g)��H2O(g)���������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ(ע��T1��T2������300 ��)��
 2H2O �� ��
2H2O �� ��