ƒøƒ⁄»›
œ¬¡–∏˜◊È¿Î◊”‘⁄÷∏∂®»‹“∫÷–ƒ‹¥Û¡øπ≤¥Êµƒ «£®°°°°£©
¢Ÿ≥£Œ¬œ¬£¨c£®H+£©/c£®OH“ª£©=1°¡10-12µƒ»‹“∫£∫K+°¢AlO2-°¢CO32-°¢Na+
¢⁄º”»Î±Ω∑”œ‘◊œ…´µƒ»‹“∫£∫K+°¢NH4+°¢Cl-°¢I“ª
¢€÷––‘»‹“∫£∫Fe3+°¢Al3+°¢NO3-°¢SO42-
¢‹ πpH ‘÷Ω±‰¿∂µƒ»‹“∫÷–£∫Al3+°¢Na+°¢SO42-°¢Cl“ª
¢›Œfi…´»‹“∫÷–£∫K+°¢Al3+°¢NO3-°¢HCO3-£Æ
¢Ÿ≥£Œ¬œ¬£¨c£®H+£©/c£®OH“ª£©=1°¡10-12µƒ»‹“∫£∫K+°¢AlO2-°¢CO32-°¢Na+
¢⁄º”»Î±Ω∑”œ‘◊œ…´µƒ»‹“∫£∫K+°¢NH4+°¢Cl-°¢I“ª
¢€÷––‘»‹“∫£∫Fe3+°¢Al3+°¢NO3-°¢SO42-
¢‹ πpH ‘÷Ω±‰¿∂µƒ»‹“∫÷–£∫Al3+°¢Na+°¢SO42-°¢Cl“ª
¢›Œfi…´»‹“∫÷–£∫K+°¢Al3+°¢NO3-°¢HCO3-£Æ
∑÷Œˆ£∫¢Ÿ≥£Œ¬œ¬£¨c£®H+£©/c£®OH“ª£©=1°¡10-12µƒ»‹“∫£¨c£®OH-£©=0.1mol/L£¨»‹“∫œ‘ºÓ–‘£ª
¢⁄º”»Î±Ω∑”œ‘◊œ…´µƒ»‹“∫£¨∫¨Fe3+£ª
¢€Fe3+‘⁄pH=4.7◊Û”“◊™ªØŒ™≥¡µÌ£ª
¢‹ πpH ‘÷Ω±‰¿∂µƒ»‹“∫£¨œ‘ºÓ–‘£ª
¢›¿Î◊”÷ƺ‰œ‡ª•¥ŸΩ¯ÀÆΩ‚£Æ
¢⁄º”»Î±Ω∑”œ‘◊œ…´µƒ»‹“∫£¨∫¨Fe3+£ª
¢€Fe3+‘⁄pH=4.7◊Û”“◊™ªØŒ™≥¡µÌ£ª
¢‹ πpH ‘÷Ω±‰¿∂µƒ»‹“∫£¨œ‘ºÓ–‘£ª
¢›¿Î◊”÷ƺ‰œ‡ª•¥ŸΩ¯ÀÆΩ‚£Æ
Ω‚¥£∫Ω‚£∫¢Ÿ≥£Œ¬œ¬£¨c£®H+£©/c£®OH-£©=1°¡10-12µƒ»‹“∫£¨c£®OH-£©=0.1mol/L£¨»‹“∫œ‘ºÓ–‘£¨∏√◊È¿Î◊”÷ƺ‰≤ª∑¥”¶£¨ƒ‹π≤¥Ê£¨π ’˝»∑£ª
¢⁄º”»Î±Ω∑”œ‘◊œ…´µƒ»‹“∫£¨∫¨Fe3+£¨”ÎI-∑¢…˙—ıªØªπ‘≠∑¥”¶£¨≤ªƒ‹π≤¥Ê£¨π ¥ÌŒÛ£ª
¢€Fe3+‘⁄pH=4.7◊Û”“◊™ªØŒ™≥¡µÌ£¨Fe3+≤ªª·¥Û¡ø¥Ê‘⁄£¨π ¥ÌŒÛ£ª
¢‹ πpH ‘÷Ω±‰¿∂µƒ»‹“∫£¨œ‘ºÓ–‘£¨Al3+°¢OH-∑¥”¶£¨‘Ú≤ªƒ‹π≤¥Ê£¨π ¥ÌŒÛ£ª
¢›Al3+°¢HCO3-¿Î◊”÷ƺ‰œ‡ª•¥ŸΩ¯ÀÆΩ‚£¨≤ªƒ‹π≤¥Ê£¨π ¥ÌŒÛ£ª
π —°C£Æ
¢⁄º”»Î±Ω∑”œ‘◊œ…´µƒ»‹“∫£¨∫¨Fe3+£¨”ÎI-∑¢…˙—ıªØªπ‘≠∑¥”¶£¨≤ªƒ‹π≤¥Ê£¨π ¥ÌŒÛ£ª
¢€Fe3+‘⁄pH=4.7◊Û”“◊™ªØŒ™≥¡µÌ£¨Fe3+≤ªª·¥Û¡ø¥Ê‘⁄£¨π ¥ÌŒÛ£ª
¢‹ πpH ‘÷Ω±‰¿∂µƒ»‹“∫£¨œ‘ºÓ–‘£¨Al3+°¢OH-∑¥”¶£¨‘Ú≤ªƒ‹π≤¥Ê£¨π ¥ÌŒÛ£ª
¢›Al3+°¢HCO3-¿Î◊”÷ƺ‰œ‡ª•¥ŸΩ¯ÀÆΩ‚£¨≤ªƒ‹π≤¥Ê£¨π ¥ÌŒÛ£ª
π —°C£Æ
µ„∆¿£∫±æÂøº≤È¿Î◊”µƒπ≤¥Ê£¨√˜»∑œ∞Â÷–µƒ–≈œ¢ «Ω‚¥±æµƒπÿº¸£¨◊¢“‚œ∞Â÷–µƒ—ıªØªπ‘≠∑¥”¶Œ™Ω‚¥µƒƒ—µ„£¨Ã‚ƒøƒ—∂»≤ª¥Û£Æ

¡∑œ∞≤·œµ¡–¥∞∏
 ‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
œ‡πÿƒø
 £¨Na+£¨HSO
£¨Na+£¨HSO £¨PO
£¨PO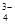 £¨MnO
£¨MnO
 £¨NO
£¨NO £¨SO
£¨SO
 £¨CO
£¨CO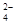 £¨Cl
£¨Cl =0.1 mol/Lµƒ»‹“∫÷–£∫Na+£¨K+£¨SO
=0.1 mol/Lµƒ»‹“∫÷–£∫Na+£¨K+£¨SO