��Ŀ����
���ʽṹ��
��֪A��B��C��D��E����Ԫ�صĺ˵������������EΪ��������Ԫ���⣬����Ƕ�����Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AԪ���������������ڲ��������2����BԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��EԪ�صģ�3�����ӵ�3d�ܼ�Ϊ�����״̬��(����ʱ��ABCDE��Ӧ��Ԫ�ط��ű�ʾ)
(1)A��һ���⻯��A2H2��������ԭ�Ӳ�ȡ���ӻ���ʽΪ________�������к���________���ļ���________�м���
(2)д��������DC�ĵ���ʽ________��Eԭ�ӵĺ�������Ų�ʽ________��
(3)��EԪ���γɵĽ����ľ����ṹ����ͼ����þ����к��н���ԭ�ӵ���ĿΪ________��
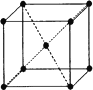
(4)������E(CO)5������ΪҺ̬���۵�Ϊ��20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ��ж�E(CO)5��������________(�������)��

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�[��ѧ��ѡ�����ʽṹ������]��15�֣�
�±�Ϊ���ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ����֪A��D��ԭ��������������
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | Aԭ������������������������ԭ����������� |
| B | ���������6�ֲ�ͬ�˶�״̬ |
| C | ����⻯���ˮ��Һ�ʼ��� |
| D | ��̬ԭ�ӵ�S�������������P��������� |
A����1��д��Cԭ�ӵĵ����Ų�ͼ____________
��2��A��B��C��D�ĵ�һ�������ɴ�С��˳��Ϊ______________
��3���õ��뷽��ʽ��ʾC������⻯���ˮ��Һ�ʼ��Ե�ԭ�� _______________
B�����չ��ۼ����ۣ�������ÿ��ԭ�ӵ�������Ӳ���������ѱ��͡���֪ABCD���ӿ��������ֲ�ͬ�ṹ�����ǵĽṹ���Դ��������ʵĽṹ�л��������
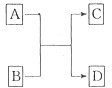
��4��ABC�Ľṹʽ��___________�������в���һ��Dԭ�ӣ����γ���λ�����á���ʾ����ABCD�ĽṹʽΪ______________������C��ԭ�ӵ��ӻ�������______________��������λ����ABCD�ĽṹʽΪ___________������Dԭ�ӵ��ӻ�����Ϊ______________��
��5��BD2�ĵ���ʽ��____________��Dԭ�ӻ��ɵȵ��ӵ�
 ����ABCD�ĽṹʽΪ________��������Cԭ�ӵ��ӻ�������______________��
����ABCD�ĽṹʽΪ________��������Cԭ�ӵ��ӻ�������______________����6����ABCD�����ֿ��ܽṹ��Bԭ�ӵ��ӻ�����__________�����ͬ������ͬ����
[��ѧ��ѡ�����ʽṹ������]��15�֣�
�±�Ϊ���ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ����֪A��D��ԭ��������������
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
A |
Aԭ������������������������ԭ����������� |
|
B |
���������6�ֲ�ͬ�˶�״̬ |
|
C |
����⻯���ˮ��Һ�ʼ��� |
|
D |
��̬ԭ�ӵ�S�������������P��������� |
���ϱ�����Ϣ������и�С�⣨����ʱ������Ӧ��Ԫ�ط��ţ�
A����1��д��Cԭ�ӵĵ����Ų�ͼ____________
��2��A��B��C��D�ĵ�һ�������ɴ�С��˳��Ϊ______________
��3���õ��뷽��ʽ��ʾC������⻯���ˮ��Һ�ʼ��Ե�ԭ�� _______________
B�����չ��ۼ����ۣ�������ÿ��ԭ�ӵ�������Ӳ���������ѱ��͡���֪ABCD���ӿ��������ֲ�ͬ�ṹ�����ǵĽṹ���Դ��������ʵĽṹ�л��������
��4��ABC�Ľṹʽ��___________�������в���һ��Dԭ�ӣ����γ���λ�����á���ʾ����ABCD�ĽṹʽΪ______________������C��ԭ�ӵ��ӻ�������______________��������λ����ABCD�ĽṹʽΪ___________������Dԭ�ӵ��ӻ�����Ϊ______________��
��5��BD2�ĵ���ʽ��____________��Dԭ�ӻ��ɵȵ��ӵ� ����ABCD�ĽṹʽΪ________��������Cԭ�ӵ��ӻ�������______________��
����ABCD�ĽṹʽΪ________��������Cԭ�ӵ��ӻ�������______________��
��6����ABCD�����ֿ��ܽṹ��Bԭ�ӵ��ӻ�����__________�����ͬ������ͬ����
 ��2013?����ģ�⣩��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X����������Y���ڲ��������ȣ�Yԭ�ӵ������������Ǵ�����������������Z��L�ǿ����к������Ķ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺
��2013?����ģ�⣩��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X����������Y���ڲ��������ȣ�Yԭ�ӵ������������Ǵ�����������������Z��L�ǿ����к������Ķ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺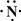 ��
��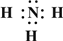

 ����ABCD�ĽṹʽΪ
����ABCD�ĽṹʽΪ