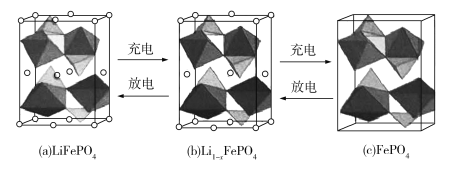
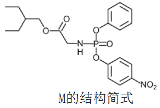
��Ŀ����
����Ŀ����¯����ʱ�ô�O2����������Ʒ�����ݲ��������ȷ���л������ɡ���ͼ1װ������ȼ�շ�ȷ���л������ʽ���õ�װ�á�

(1)ȼ�չ���CuO��������_____��
(2)��ʵ������ȡ��Ʒֻ��C��H��O����Ԫ���е����ֻ����֣�ȷ��ȡ1.48g��Ʒ������ַ�Ӧ��A����������3.52g��B����������1.8g�������Ʒ��ʵ��ʽΪ_____��
(3)�������Dzⶨ����Է�������������ͼ2��ʾ������ͼ������л������Է�������Ϊ_____��

(4)��ֻ����A��ʵ��ʽ�ܷ�ȷ�������ʽ____������������������������
(5)�����ʵĺ������ͼ��ͼ3��ʾ������ṹ��ʽΪ____��

���𰸡�ʹ�л�����������ΪCO2��H2O C4H10O 74 �� CH3CH2OCH2CH3
��������
����ʵ��ԭ���Dzⶨһ���������л�����ȫȼ��ʱ����CO2��H2O����������ȷ���Ƿ�����C��H��O�ĸ����ȣ�������ʽ���������O2�������ӣ���Ҫ�dz�H2O������ȷ��װ�õ������ǽ����ǰ�ᡣD�����ɵ������к���ˮ������Ӧ��ͨ��C�е�Ũ��������E�е�¯����ʱ�ô�������������Ʒ�����ɶ�����̼��ˮ������һ����̼���ɣ���E��CuO����CO��һ����Ӧ���ɶ�����̼��Ȼ��ֱ�ͨ��B������ˮ����A�����ն�����̼���У����ݲ���������ƶ��л������ɣ��ݴ˷������
(1)��һ����̼��������ͭ��Ӧ���ɱ������ɶ�����̼�����ʿ�֪��CuO�������ǰ��л��ﲻ��ȫȼ�ղ�����COת��ΪCO2���ʴ�Ϊ��ʹ�л�����������ΪCO2��H2O��
(2)A����������3.52g��˵��������3.52g������̼���ɵ�̼Ԫ�ص�����,3.52g��![]() ��100%=0.96g��B����������1.8g��˵��������1.8gˮ���ɵ���Ԫ�ص�������1.8g��
��100%=0.96g��B����������1.8g��˵��������1.8gˮ���ɵ���Ԫ�ص�������1.8g��![]() ��100%=0.2g���Ӷ����Ƴ�����Ԫ�ص�����Ϊ��1.48g-0.96g-0.2g=0.32g����ʵ��ʽΪCXHYOZ����X��Y��Z=
��100%=0.2g���Ӷ����Ƴ�����Ԫ�ص�����Ϊ��1.48g-0.96g-0.2g=0.32g����ʵ��ʽΪCXHYOZ����X��Y��Z=![]() ��
��![]() ��
��![]() =4��10��1����ʵ��ʽΪ��C4H10O���ʴ�Ϊ��C4H10O��
=4��10��1����ʵ��ʽΪ��C4H10O���ʴ�Ϊ��C4H10O��
(3)������ͼ��֪���л������Է�������Ϊ74���ʴ�Ϊ��74��
(4)���л����ʵ��ʽΪ��C4H10O�����Է���ʽΪ(C4H10O)n����Է�������Ϊ74n�����л������Է�������Ϊ74����n=1�����Է���ʽΪC4H10O���ʴ�Ϊ���ܣ�
(5)�ɸ����ʵĺ������ͼ��֪�����л����к��жԳƵ�-CH3���ԳƵ�-CH2-�Լ�C-O-C���ʸ��л���Ľṹ��ʽΪCH3CH2OCH2CH3���ʴ�Ϊ��CH3CH2OCH2CH3��