��Ŀ����
��14�֣����Ը������������Ĺ�ҵ��ҺΪԭ�������������Ĺ�������(���ֲ�����������)��
��.�ӷ�Һ���ᴿ���ᾧ��FeSO4��7 H2O��
H2O��
��.��FeSO4��7H2O���Ƴ���Һ��
��.FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3����Һ��
��.����Һ���ˣ���90 ����ˮϴ�ӳ����������õ�FeCO3���塣
��.����FeCO3���õ�Fe2O3���塣
��֪��NH4HCO3����ˮ�зֽ⡣
(1)���У�����������м��ȥ��Һ�е�Fe3�����÷�Ӧ�����ӷ���ʽ��_________________��
(2)���У����һ�����ᣬ��������� �����û�ѧƽ��ԭ���������������________________________________________________________________________��
(3)���У�����FeCO3�����ӷ���ʽ��___________________________________________
��FeCO3��Һ��ʱ�䱩¶�ڿ����У����в��ֹ�������Ϊ���ɫ���ñ仯�Ļ�ѧ����ʽ��_______________________________________��
(4)���У�ͨ������SO���жϳ����Ƿ�ϴ�Ӹɾ�������SO�IJ�����__________
________________________________________________________________________��
(5)��֪����FeCO3�Ļ�ѧ����ʽ��4FeCO3��O22Fe2O3��4CO2��������464.0 kg��FeCO3���õ�316.8 kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������________kg��(Ħ������/g��mol��1��FeCO3 116 Fe2O3 160 FeO 72)
kg��FeCO3���õ�316.8 kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������________kg��(Ħ������/g��mol��1��FeCO3 116 Fe2O3 160 FeO 72)
��.�ӷ�Һ���ᴿ���ᾧ��FeSO4��7
 H2O��
H2O����.��FeSO4��7H2O���Ƴ���Һ��
��.FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3����Һ��
��.����Һ���ˣ���90 ����ˮϴ�ӳ����������õ�FeCO3���塣
��.����FeCO3���õ�Fe2O3���塣
��֪��NH4HCO3����ˮ�зֽ⡣
(1)���У�����������м��ȥ��Һ�е�Fe3�����÷�Ӧ�����ӷ���ʽ��_________________��
(2)���У����һ�����ᣬ��������� �����û�ѧƽ��ԭ���������������________________________________________________________________________��
(3)���У�����FeCO3�����ӷ���ʽ��___________________________________________
��FeCO3��Һ��ʱ�䱩¶�ڿ����У����в��ֹ�������Ϊ���ɫ���ñ仯�Ļ�ѧ����ʽ��_______________________________________��
(4)���У�ͨ������SO���жϳ����Ƿ�ϴ�Ӹɾ�������SO�IJ�����__________
________________________________________________________________________��
(5)��֪����FeCO3�Ļ�ѧ����ʽ��4FeCO3��O22Fe2O3��4CO2��������464.0
 kg��FeCO3���õ�316.8 kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������________kg��(Ħ������/g��mol��1��FeCO3 116 Fe2O3 160 FeO 72)
kg��FeCO3���õ�316.8 kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������________kg��(Ħ������/g��mol��1��FeCO3 116 Fe2O3 160 FeO 72)
��

��ϰ��ϵ�д�
�����Ŀ
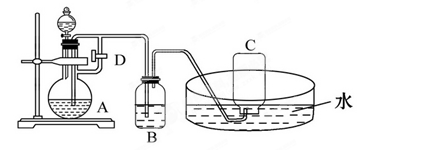
 ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽΪ____________________________________________________��
ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽΪ____________________________________________________��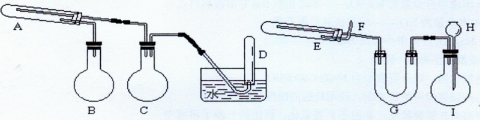

 �������ӷ�Ӧ����ʽ
�������ӷ�Ӧ����ʽ ________________________�����백ˮҪ������ԭ����___________________________��
________________________�����백ˮҪ������ԭ����___________________________�� _
_ _________________
_________________ _______________________________________________��
_______________________________________________�� ��ȷ�����ղ����Ľ��ƫ�����������ԭ�������___________________________
��ȷ�����ղ����Ľ��ƫ�����������ԭ�������___________________________
 O �� FeCl3��6H2O�����ʵ���֮�����ӦΪ ���ڴ������£�������Ԫ���Ƿ������ȫ��ʵ������� ��
O �� FeCl3��6H2O�����ʵ���֮�����ӦΪ ���ڴ������£�������Ԫ���Ƿ������ȫ��ʵ������� ��