��Ŀ����
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
(1)W��Ԫ�����ڱ��е�λ����_____________��
(2) 2.24 L(��״��)XQ3��200 mL 1 moL/L QXY3����Һ���պ�������Һ������Ũ�ȴӴ�С��˳����___________________��
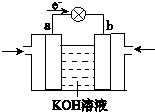
(3)WQ4Y��Y2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ����ͼ��ʾ��a���ĵ缫��Ӧʽ��______________________��
(1)W��Ԫ�����ڱ��е�λ����_____________��
(2) 2.24 L(��״��)XQ3��200 mL 1 moL/L QXY3����Һ���պ�������Һ������Ũ�ȴӴ�С��˳����___________________��
(3)WQ4Y��Y2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ����ͼ��ʾ��a���ĵ缫��Ӧʽ��______________________��

(4)��֪��
W(s)+Y2(g)=WY2(g) ��H= -393��5 kJ/mol
WY(g)+1/2Y2(g)=WY2(g) ��H= -283.0 kJ/mol
24g W��һ������Y2��Ӧ���ų�����362��5 kJ�����ò�������ʵ���֮����__________��
(5)X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��____________________________��
W(s)+Y2(g)=WY2(g) ��H= -393��5 kJ/mol
WY(g)+1/2Y2(g)=WY2(g) ��H= -283.0 kJ/mol
24g W��һ������Y2��Ӧ���ų�����362��5 kJ�����ò�������ʵ���֮����__________��
(5)X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��____________________________��
(1)�ڶ����ڵڢ�A��
(2)c(NO3-)�� c(H+) ��c(NH4+)�� c(OH-)
(3)CH3OH-6e- + 8OH- == CO32- + 6H2O
(4)n(CO2):n(CO)=1:3
(5)Na3N + 4H2O == 3NaOH + NH3��H2O
(2)c(NO3-)�� c(H+) ��c(NH4+)�� c(OH-)
(3)CH3OH-6e- + 8OH- == CO32- + 6H2O
(4)n(CO2):n(CO)=1:3
(5)Na3N + 4H2O == 3NaOH + NH3��H2O

��ϰ��ϵ�д�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д�
�����Ŀ
Q��W��X��Y��Z��ԭ��������������Ķ�����Ԫ�أ�X����ɫ��Ӧ�ʻ�ɫ��QԪ�ص�ԭ�����������������ڲ��������2����W��Z������������ͬ��Z�ĺ˵������W��2����Ԫ��Y�ĺϽ����ճ�������ʹ�ù㷺�Ľ�������֮һ������˵����ȷ���ǣ�������
| A���⻯���ȶ��ԣ�Z��W | B��ԭ�Ӱ뾶�Ĵ�С˳��rX��rY��rQ��rW | C��Ԫ��Q��Z���γ�QZ2�͵Ĺ��ۻ����� | D��X��Y������������ˮ����֮�䲻�ܷ�����Ӧ |
 Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
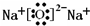
 Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������W��Q��1��4�γɵĻ�����A��һ����Ҫ��Դ��W��Y��X��Y��ɵĻ������dz����Ĵ�����Ⱦ�Y��Z��1��1�γɵ����ӻ�����B����Ħ������Ϊ78g?mol-1����ش��������⣮
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������W��Q��1��4�γɵĻ�����A��һ����Ҫ��Դ��W��Y��X��Y��ɵĻ������dz����Ĵ�����Ⱦ�Y��Z��1��1�γɵ����ӻ�����B����Ħ������Ϊ78g?mol-1����ش��������⣮