��Ŀ����
����Ŀ����������ʾ�����İ����������ڳ����¿ɺϳ�����ɰ(��Ҫ�ɷ�ΪNH4Cl)��ijС�������ɰ��������̽����
��.����ɰ��ʵ�����Ʊ�

(1)Cװ����ʢ�ż�ʯ�ҵ���������Ϊ____________________��
(2)Ϊʹ������������D�г�ֻ�ϲ���Ӧ������װ�õ�����˳��Ϊa��d��c��____��_____��j��i��h��g��b��
(3)װ��D�������������⣬���в���֮��Ϊ______________________��
(4)���鰱����������Ӧ������ɰ����ʱ����������ˮ��ϡHNO3��AgNO3��Һ����ɫʯ����ֽ�⣬����Ҫ���Լ�Ϊ_______________________��
��.��Ȼ����ɰ��NH4Cl���ȵIJⶨ(���ʲ�Ӱ��NH4Cl���Ȳⶨ)
��֪��2NH4Cl+3CuO![]() 3Cu+2HCl��+N2��+3H2O��
3Cu+2HCl��+N2��+3H2O��
���裺��ȷ��ȡ1.19g����ɰ���ڽ�����ɰ������������ͭ��ϼ���(װ������)��

(1)���Ӻ��������װ�õ�������ʱ���Ƚ�H��K��װ������ˮ��Ȼ�����G��____�������������á�
(2)װ��H������___________________________��
(3)ʵ�������װ��I����0.73g������Ȼ����ɰ��NH4Cl����������Ϊ_________________��
(4)����K����������ⶨNH4Cl���ȣ�����������Һ�������Ͳ��Һ��ʱ�����ⴿ��______(�ƫ�ߡ����� ��Ӱ�족��ƫ�͡�)
���𰸡� ����� e f ��β������װ�� ��������Ũ��Һ ����ĩ��������ð����ֹͣ���ȣ����������γ�һ��ˮ�� ���շ�Ӧ������ˮ��������ֹ��HCl�ⶨ��ɸ��� 89.9% ƫ��
��������(1)Cװ����ʢ�ż�ʯ�ҵ���������Ϊ����ܣ���ȷ�𰸣�����ܡ�
(2)��ȡ����������˳��Ϊadc����ȡ����������˳��Ϊbghij,���ǵ��������ܶȱȿ����������ܶȱȿ���С����������e�ڽ���������f�ڽ����������������γ������������������ڶ��߳�ֻ�ϣ�ͬʱע��ȷ������˳��ʱ�����������Ǵ�������ģ�����������˳��Ϊa��d��c��e��f��j��i��h��g��b����ȷ�𰸣�e ��f��
(3) װ��D��������Ӧ�������Ȼ�泥��Ȼ��Ϊ����С����������̫ϸ������������������ܣ���Ϊ�����ж�����Ҫ��β������װ�ã����Բ���֮��Ϊ����β������װ�ã���ȷ�𰸣���β������װ�á�
(4) ��������Ȼ���е�笠�������Ҫ����������Һ�ͺ�ɫʯ����ֽ��������������Ҫ����ˮ����������ϡ���ᣬ���Ի���Ҫ���Լ�Ϊ��������Ũ��Һ����ȷ�𰸣���������Ũ��Һ��
��.(1)���Ӻ��������װ�õ�������ʱ���Ƚ�H��K��װ������ˮ��Ȼ�����G������ĩ��������ð����ֹͣ���ȣ����������γ�һ��ˮ���������������ã���ȷ�𰸣�����ĩ��������ð����ֹͣ���ȣ����������γ�һ��ˮ����
(2)����������ͨ��װ��H�е�Ũ���ᣬ�ܹ����ջ�������е�ˮ��������ֹ��HCl�ⶨ��ɸ��ţ���Сʵ������ȷ�𰸣����շ�Ӧ������ˮ��������ֹ��HCl�ⶨ��ɸ��š�
(3) װ��I����Ϊ�Ȼ��������������0.73g�Ȼ�������ʵ���Ϊ0.02mol�����ݷ�Ӧ2NH4Cl+3CuO![]() 3Cu+2HCl��+N2��+3H2O��֪�������Ȼ�淋���Ϊ0.02mol������Ϊ0.02��53.5=1.07g������Ȼ����ɰ��NH4Cl����������Ϊ1.07/1.19��100%=89.9%����ȷ�𰸣�89.9%��
3Cu+2HCl��+N2��+3H2O��֪�������Ȼ�淋���Ϊ0.02mol������Ϊ0.02��53.5=1.07g������Ȼ����ɰ��NH4Cl����������Ϊ1.07/1.19��100%=89.9%����ȷ�𰸣�89.9%��
(4)����������Һ�������Ͳ��Һ��ʱ�����ⶨ���������ƫС�����ݷ�Ӧ2NH4Cl+3CuO![]() 3Cu+2HCl��+N2��+3H2O��֪���Ȼ�淋�����ƫС�����ⴿ��ƫ�ͣ���ȷ�𰸣�ƫ�͡�
3Cu+2HCl��+N2��+3H2O��֪���Ȼ�淋�����ƫС�����ⴿ��ƫ�ͣ���ȷ�𰸣�ƫ�͡�

����Ŀ��ijѧ����0.2000mol/L�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ���������: ���ñ���Һ��ϴ�ζ���2-3 �Σ�ȡ��NaOH ��Һע���ʽ�ζ�������0���̶������������̶��õζ��ܲ�ʹ�ζ��ܼ������Һ����������Һ������0������0���̶������£������¶���������ȡ20.00mL����Һע���徻����ƿ�У�������3�η�̪��Һ�����ñ�Һ�ζ����յ������µζ���Һ����������ظ����ϵζ�����2-3 ����
��ش���������:
��1���������У�����ȡ����ĵζ��ܼ��첿����������ȡҺ����ǰ������ʧ����ⶨ���____(����ƫ��������ƫС������������)��
��2���жϵ���ζ�·���������____________��
��3��������ʵ�����ݼ�¼��
�ζ����� | �������(mL) | NaOH��Һ�������(mL) | |
�ζ�ǰ | �ζ��� | ||
1 | 20.00 | 0.00 | 21.10 |
2 | 20.00 | 0.00 | 19.40 |
3 | 20.00 | 0.00 | 19.32 |
���ϱ����Կ�������1�εζ���¼��NaOH��Һ������Զ��ں����ε����������ܵ�ԭ����__________
A.NaOH ��Һ����ʱ��������в��ֱ���
B.��ƿ�ô���Һ��ϴ
C.����NaOH ��Һ���õ�ҩƷ�л���KOH����
D.�ζ�����ʱ�����Ӷ���
��4�������ϱ���¼���ݣ�ͨ������ɵã�������Ũ��Ϊ_____mol/L��
��5�������£���0.100mol/LNaOH��Һ�ֱ�ζ�20.00mL0.100mol/L������ʹ������ζ�������ͼ��ʾ������˵����ȷ����_________
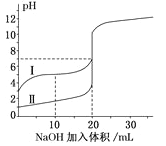
A.V(NaOH)=20mLʱ��c(Cl-)=c(CH3COO-)
B.I��ʾ���ǵζ����������
c.pH=7ʱ���ζ���������V(NaOH)С��20mL
D.V(NaOH)=10mLʱ��������Һ��:c(Na+)>c(CH3COO-)>c(H+)>c(OH-)
����Ŀ����ȥ���������ڵ����ʣ������Լ��Ͳ�������ȷ����
ѡ�� | ����ӵ����� | �Լ� | ���� |
A | Cl2��HCl�� | ����ʳ��ˮ | ϴ�� |
B | NaBr��Һ��NaI�� | Cl2 | ϴ�� |
C | Cl2��H2O�� | ��ʯ��(CaO��NaOH) | ϴ�� |
D | ���ۣ����ۣ� | ϡ���� | ���� |
A.AB.BC.CD.D


