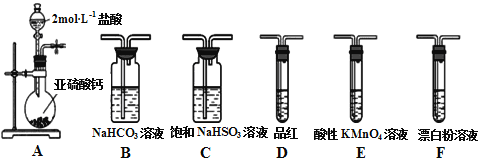
��Ŀ����
����Ŀ�����������������ؾ���(K3[Fe(C2O4)3]��3H2O)������ˮ���������Ҵ����������Թ����У������¼������ֽ⡣���������������Ʊ�����������������и��⣺
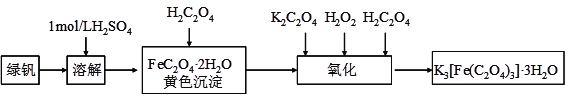
��1���ܽ�ʱ��ϡ�����������__________________________
��2�����ܽ�Һ����һ�����IJ��������У��γɻ�ɫ���������ˣ�ϴ�ӡ�
������ͼ�ǹ���װ��ͼ��ָ��ͼ���������Դ���һ����_____________________����һ����©�����¼��û�н������ܵ��ձ���
�����ȷ�������Ѿ�ϴ�Ӹɾ�____________________________________��
��3�����������г����Ͻ����⣬ά���¶���40�����ң�ԭ����___________________________��
��4��д�������������������������ص����ӷ���ʽ__________________________________��
��5�����������������ؾ�������¼��ɷֽ��������ֲ����Ρ�CO2�ȡ�д���÷ֽⷴӦ�Ļ�ѧ����ʽ______________________��
���𰸡� ����Fe2+��ˮ�� δʹ�ò��������� ȡ����ϴ��Һ�������еμ������ữ���Ȼ�����Һ����������ɫ����������ϴ�Ӹɾ� �¶�̫�ͷ�Ӧ���������¶ȸ߹��������ֽ� 2FeC2O4��2H2O+H2O2+H2C2O4+3C2O42-=2[Fe(C2O4)3]3-+6H2O 2K3[Fe(C2O4)3]��3H2O![]() 3K2C2O4+2FeC2O4+2CO2��+6H2O
3K2C2O4+2FeC2O4+2CO2��+6H2O
�������������������1������������ˮ������ԣ�����������Է�ֹˮ�⣻��2����������Ҫ�ò�����������������ϴ�Ӹɾ���ϴ��Һ�в���SO42-�����Ը���ϴ��Һ���Ƿ���SO42-�ж��Ƿ�ϴ�Ӹɾ�����3���¶�̫�ͷ�Ӧ���������¶ȸ߹��������ֽ�����4����������FeC2O4��2H2O��H2O2��H2C2O4��K2C2O4�������������(��)�������5�����������(��)��ؾ�������¼��ɷֽ�����CO2������������ԭ��Ӧ���ɣ����ɵIJ�������K2C2O4��FeC2O4��
��������1������������ˮ������ԣ�������������Fe2+��ˮ������2���ٹ�����Ҫ�ò������������������Դ�����δʹ�ò�������������ȷ�������Ѿ�ϴ�Ӹɾ��ķ����ǣ�ȡ����ϴ��Һ�������еμ������ữ���Ȼ�����Һ����������ɫ����������ϴ�Ӹɾ�����3���¶�̫�ͷ�Ӧ���������¶ȸ߹��������ֽ⣬����ά���¶���40����������4����������FeC2O4��2H2O��H2O2��H2C2O4��K2C2O4�������������(��)��أ���Ӧ����ʽ��2FeC2O4��2H2O+H2O2+H2C2O4+3C2O42-=2[Fe(C2O4)3]3-+6H2O����5�����������(��)��ؾ�������¼��ɷֽ�����CO2������������ԭ��Ӧ���ɣ����ɵIJ�������K2C2O4��FeC2O4���÷ֽⷴӦ�Ļ�ѧ����ʽ��2K3[Fe(C2O4)3]��3H2O![]() 3K2C2O4+2FeC2O4+2CO2��+6H2O��
3K2C2O4+2FeC2O4+2CO2��+6H2O��

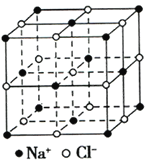
����Ŀ�������±��е���Ϣ�����ش����⣺
Ԫ�� | Ԫ�ػ���Ԫ����ɵ��������� |
A | ��������Ȼ����Ӳ�����ȼ�ղ�����������ʹ����ʯ��ˮ����ǡ� |
B | ԭ�������������Ǵ������������� |
C | ������ɫ��ӦΪ��ɫ��������ȼ�����ɵ���ɫ���� |
D | �����ڿ��������������� |
E | ����Ϊ����ɫ��ȼ�ղ���������Ư���ԡ� |
F | ��ͬ�����������ܶ���С |
��1���õ���ʽд���γ�F2E�Ĺ���__________________��
��2��д��CBF���ʵĵ���ʽ______________��
��3��C2B2�����д��ڻ�ѧ����������___________ ��1 molC2B2������AB2��Ӧת�Ƶĵ�����Ϊ_______��
��4����B��C��E�γɵļ����Ӱ뾶�ɴ�С�Ĺ�ϵ��______________�������ӷ��ű�ʾ����