��Ŀ����
ij��A 0.2 mol�������г��ȼ�պ����ɻ�����B��C��1.2 mol��������������⣺
(1)��A�ķ���ʽ��_________��
(2)��ȡһ��������A���ȼ�պ�����B��C��3 mol������_________g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״���µ�����_________ L��
(3)����A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�����A�Ľṹ��ʽ��___________��
(4)����A��ʹ��ˮ��ɫ���ڴ�����������H2�����ӳɷ�Ӧ������2��2-�������飬����A��������_________���ṹ��ʽ��_________��
(5)��A������̼ԭ�ӵ�A��ϩ��ͬϵ���ͬ���칹�干��_________�֡�
(1)C6H12 (2)42 100.8 (3)
(4)3��3-����-1-��ϩ 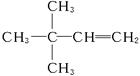 (5)3
(5)3
����:
(1)�������������A�ķ���ʽΪC6H12��(3)C6H12������ϩ����Ҳ�����ǻ���������Ϊ��A����ʹ��ˮ��ɫ��˵����A���ڻ�����������C6H12Ϊ�����飬��ṹ��ʽΪ ��
��
(4)����A��ʹ��ˮ��ɫ��˵��C6H12Ϊϩ��������������Ϊ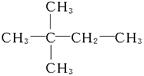 ������AӦΪ
������AӦΪ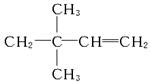 ������Ϊ3��3��-����-1-��ϩ��
������Ϊ3��3��-����-1-��ϩ��
(5)ͬ���칹���У���ϩ������
CH3��CH2��CH==CH2��CH3��CH==CH��CH3��![]() 3�֡�
3�֡�

 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�