��Ŀ����
�����ѧ���������ɫ���ɡ����룺�ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��ʹ�����е�CO2ת��Ϊ������ȼ�ϼ״�������ɫ���ɡ����뼼���������£�

��1���ںϳ����У�����2.2kgCO2������H2ǡ����ȫ��Ӧ��������̬��ˮ�ͼ״����ɷų�2473.5kJ����������д���ϳ����з�����Ӧ���Ȼ�ѧ����ʽ______��
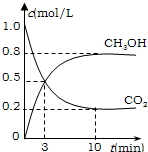
��2���������ϳɷ�Ӧ����һ���Ŀ����ԣ���ƽ���ƶ�ԭ������������������ԭ������ת������ʵ�������в���300��C���¶ȣ���ԭ����______��
�ڡ���ɫ���ɡ����뼼�������г��������ʺ������ġ�ѭ�����á������������������֡�ѭ�����á��ij�̼�����Һ�⣬������______��
�����д�ʩ����ʹn��CH3OH��/n��CO2���������______��
A�������¶� B������He��g����ʹ��ϵѹǿ����
C����H2O��g������ϵ�з��� D���ٳ���1molCO2��3molH2
��3���״�������ȼ�ϵ�أ�д������������Ϊ����ʵļ״�ȼ�ϵ�ظ�����Ӧʽ______��������ת�Ƶ����ʵ���Ϊ______ʱ���μӷ�Ӧ�������������6.72L����״���£���
��4�������£�0.1mol/L KHCO3��Һ��pH����8������Һ�и�������Ũ���ɴ�С��˳��Ϊ��______��

��1���ںϳ����У�����2.2kgCO2������H2ǡ����ȫ��Ӧ��������̬��ˮ�ͼ״����ɷų�2473.5kJ����������д���ϳ����з�����Ӧ���Ȼ�ѧ����ʽ______��
��2���������ϳɷ�Ӧ����һ���Ŀ����ԣ���ƽ���ƶ�ԭ������������������ԭ������ת������ʵ�������в���300��C���¶ȣ���ԭ����______��
�ڡ���ɫ���ɡ����뼼�������г��������ʺ������ġ�ѭ�����á������������������֡�ѭ�����á��ij�̼�����Һ�⣬������______��
�����д�ʩ����ʹn��CH3OH��/n��CO2���������______��
A�������¶� B������He��g����ʹ��ϵѹǿ����
C����H2O��g������ϵ�з��� D���ٳ���1molCO2��3molH2
��3���״�������ȼ�ϵ�أ�д������������Ϊ����ʵļ״�ȼ�ϵ�ظ�����Ӧʽ______��������ת�Ƶ����ʵ���Ϊ______ʱ���μӷ�Ӧ�������������6.72L����״���£���
��4�������£�0.1mol/L KHCO3��Һ��pH����8������Һ�и�������Ũ���ɴ�С��˳��Ϊ��______��
��1��2.2kgCO2�����ʵ���Ϊ��
=50mol������1mol�״��ų�����Ϊ��
=49.47kJ/mol����ϳ����з�����Ӧ���Ȼ�ѧ����ʽΪ��
CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.47kJ?mol-1���ʴ�Ϊ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.47kJ?mol-1��
��2����ʵ�������в���300��C���¶ȣ�����Ϊ����300��Cʱ�������������Ӧ������죬�ʲ��ô��¶ȣ��ʴ�Ϊ�����ǵ������Ĵ����ԣ��ӿ췴Ӧ���ʣ�
�ڴӷ�Ӧ�����������ֽ����Ҫ����ˮ���������ϳ����¶���300��C���ʷ�����״���ˮ������ѭ�����ã��ʴ�Ϊ������ˮ������
��ʹn��CH3OH��/n��CO2������ƽ��Ӧ������Ӧ�����ƶ��������¶ȣ�ƽ�������ƶ������뺤������Ӧ��ϵŨ�Ȳ��䣬��ƽ����Ӱ�죻��H2O��g������ϵ�з��룬������������Ũ�ȣ�ƽ��������У��ٳ���1molCO2��3molH2���൱�ڼ�ѹ��ƽ��������У�ֻ��CD���ϣ��ʴ�Ϊ��CD��
��3��������ȼ�ϼ״�ʧ���ӷ���������Ӧ�����ǵ���ʵIJ��뷴Ӧ���ʵ缫��ӦʽΪ��CH3OH+8OH?-6e-�TCO32-+6H2O��
n��O2��=
=0.3mol��1molO2��Ӧת��4mol���ӣ���0.3molO2ת��1.2mol��
�ʴ�Ϊ��CH3OH+8OH?-6e-�TCO32-+6H2O��1.2mol��
��4������̼�����ˮ�⣬����c��K+����c��HCO3-��������ˮ�������ģ�����c��HCO3-����c��OH-������Һ�Լ��ԣ�����c��OH-����c��H+����
��������Դ���������棬һ��ˮ�ĵ��룬����̼������ĵ��룬��c��H+����c��CO32-�����ɴ˿�֪����Ũ�ȴ�С��ϵΪ��
c��K+����c��HCO3-����c��OH-����c��H+����c��CO32-����
�ʴ�Ϊ��c��K+����c��HCO3-����c��OH-����c��H+����c��CO32-����
| 2200g |
| 44g/mol |
| 2473.5kJ |
| 50mol |
CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.47kJ?mol-1���ʴ�Ϊ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.47kJ?mol-1��
��2����ʵ�������в���300��C���¶ȣ�����Ϊ����300��Cʱ�������������Ӧ������죬�ʲ��ô��¶ȣ��ʴ�Ϊ�����ǵ������Ĵ����ԣ��ӿ췴Ӧ���ʣ�
�ڴӷ�Ӧ�����������ֽ����Ҫ����ˮ���������ϳ����¶���300��C���ʷ�����״���ˮ������ѭ�����ã��ʴ�Ϊ������ˮ������
��ʹn��CH3OH��/n��CO2������ƽ��Ӧ������Ӧ�����ƶ��������¶ȣ�ƽ�������ƶ������뺤������Ӧ��ϵŨ�Ȳ��䣬��ƽ����Ӱ�죻��H2O��g������ϵ�з��룬������������Ũ�ȣ�ƽ��������У��ٳ���1molCO2��3molH2���൱�ڼ�ѹ��ƽ��������У�ֻ��CD���ϣ��ʴ�Ϊ��CD��
��3��������ȼ�ϼ״�ʧ���ӷ���������Ӧ�����ǵ���ʵIJ��뷴Ӧ���ʵ缫��ӦʽΪ��CH3OH+8OH?-6e-�TCO32-+6H2O��
n��O2��=
| 6.72L |
| 22.4L/mol |
�ʴ�Ϊ��CH3OH+8OH?-6e-�TCO32-+6H2O��1.2mol��
��4������̼�����ˮ�⣬����c��K+����c��HCO3-��������ˮ�������ģ�����c��HCO3-����c��OH-������Һ�Լ��ԣ�����c��OH-����c��H+����
��������Դ���������棬һ��ˮ�ĵ��룬����̼������ĵ��룬��c��H+����c��CO32-�����ɴ˿�֪����Ũ�ȴ�С��ϵΪ��
c��K+����c��HCO3-����c��OH-����c��H+����c��CO32-����
�ʴ�Ϊ��c��K+����c��HCO3-����c��OH-����c��H+����c��CO32-����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �¹�����˹��˾�ɹ����������ü״�����������ȼ�ϵ�ع��գ���ԭ����ͼ1��ʾ����۲��ͼ�ش�
�¹�����˹��˾�ɹ����������ü״�����������ȼ�ϵ�ع��գ���ԭ����ͼ1��ʾ����۲��ͼ�ش�

 Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
 CH3OH(g)��CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)��CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��