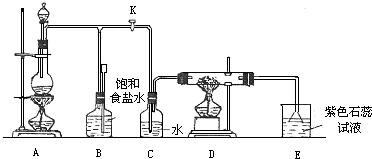
��Ŀ����
��2009?����ģ�⣩��ʯ�ͻ�����Ʒ����ѡһ������̼����ΪΨһ̼Դ���й�����Ϊԭ�Ϻϳ�
�������Ҷ�������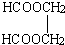 ��д��������Ӧ�Ļ�ѧ����ʽ�����ɲ�ע����Ӧ������
��д��������Ӧ�Ļ�ѧ����ʽ�����ɲ�ע����Ӧ������
��֪��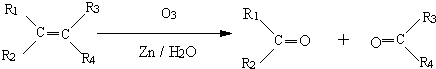 ��R1��R2��R3��R4���ɴ���������Ҳ�ɴ�����ԭ�ӣ�
��R1��R2��R3��R4���ɴ���������Ҳ�ɴ�����ԭ�ӣ�
��1��
��2��
��3��
��4��
��5��
��6��
��7��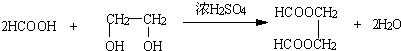
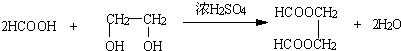 ��
��
�������Ҷ�������
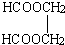 ��д��������Ӧ�Ļ�ѧ����ʽ�����ɲ�ע����Ӧ������
��д��������Ӧ�Ļ�ѧ����ʽ�����ɲ�ע����Ӧ��������֪��
 ��R1��R2��R3��R4���ɴ���������Ҳ�ɴ�����ԭ�ӣ�
��R1��R2��R3��R4���ɴ���������Ҳ�ɴ�����ԭ�ӣ���1��
CH2CH=CH2
CH3CHO+HCHO
| O3 |
| Zn/H2O |
CH2CH=CH2
CH3CHO+HCHO
��| O3 |
| Zn/H2O |
��2��
2HCHO+O2
2HCOOH
| ���� |
2HCHO+O2
2HCOOH
��| ���� |
��3��
CH3CHO+H2
CH3CH2OH
| Ni |
CH3CHO+H2
CH3CH2OH
��| Ni |
��4��
CH3CH2OH
CH2=CH2��+H2O
| Ũ���� |
| 170�� |
CH3CH2OH
CH2=CH2��+H2O
��| Ũ���� |
| 170�� |
��5��
CH2=CH2��+Br2��CH3BrCH2Br
CH2=CH2��+Br2��CH3BrCH2Br
����6��
CH3BrCH2Br+2H2O
HOCH2CH2OH+2HCl
| NaOH |
CH3BrCH2Br+2H2O
HOCH2CH2OH+2HCl
��| NaOH |
��7��
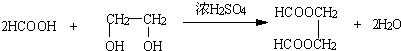
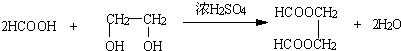
�������ϳ�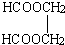 �������Ʊ�HCOOH��HOCH2CH2OH������CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO��HCHO������ΪHCOOH��CH3CHO����ԭΪCH3CH2OH��Ȼ��CH3CH2OH������ȥ��Ӧ����CH2=CH2��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br��ˮ�������HOCH2CH2OH�����߷���������Ӧ������
�������Ʊ�HCOOH��HOCH2CH2OH������CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO��HCHO������ΪHCOOH��CH3CHO����ԭΪCH3CH2OH��Ȼ��CH3CH2OH������ȥ��Ӧ����CH2=CH2��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br��ˮ�������HOCH2CH2OH�����߷���������Ӧ������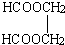 ��
��
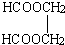 �������Ʊ�HCOOH��HOCH2CH2OH������CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO��HCHO������ΪHCOOH��CH3CHO����ԭΪCH3CH2OH��Ȼ��CH3CH2OH������ȥ��Ӧ����CH2=CH2��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br��ˮ�������HOCH2CH2OH�����߷���������Ӧ������
�������Ʊ�HCOOH��HOCH2CH2OH������CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO��HCHO������ΪHCOOH��CH3CHO����ԭΪCH3CH2OH��Ȼ��CH3CH2OH������ȥ��Ӧ����CH2=CH2��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br��ˮ�������HOCH2CH2OH�����߷���������Ӧ������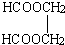 ��
������⣺�ϳ�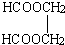 �������Ʊ�HCOOH��HOCH2CH2OH������CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO��HCHO������ΪHCOOH��CH3CHO����ԭΪCH3CH2OH��Ȼ��CH3CH2OH������ȥ��Ӧ����CH2=CH2��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br��ˮ�������HOCH2CH2OH�����߷���������Ӧ������
�������Ʊ�HCOOH��HOCH2CH2OH������CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO��HCHO������ΪHCOOH��CH3CHO����ԭΪCH3CH2OH��Ȼ��CH3CH2OH������ȥ��Ӧ����CH2=CH2��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br��ˮ�������HOCH2CH2OH�����߷���������Ӧ������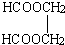 ��
��
��1��CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO����Ӧ�ķ���ʽΪCH2CH=CH2
CH3CHO+HCHO��
�ʴ�Ϊ��CH2CH=CH2
CH3CHO+HCHO��
��2��HCHO������ΪHCOOH������ʽΪ2HCHO+O2
2HCOOH���ʴ�Ϊ��2HCHO+O2
2HCOOH��
��3��CH3CHO����ԭΪCH3CH2OH������ʽΪCH3CHO+H2
CH3CH2OH���ʴ�Ϊ��CH3CHO+H2
CH3CH2OH��
��4��CH3CH2OH������ȥ��Ӧ����CH2=CH2������ʽΪCH3CH2OH
CH2=CH2��+H2O��
�ʴ�Ϊ��CH3CH2OH
CH2=CH2��+H2O��
��5��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br������ʽΪCH2=CH2��+Br2��CH3BrCH2Br��
�ʴ�Ϊ��CH2=CH2��+Br2��CH3BrCH2Br��
��6��CH3BrCH2Brˮ�������HOCH2CH2OH������ʽΪCH3BrCH2Br+2H2O
HOCH2CH2OH+2HCl��
�ʴ�Ϊ��CH3BrCH2Br+2H2O
HOCH2CH2OH+2HCl��
��7��HCOOH��HOCH2CH2OH����������Ӧ������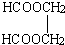 ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ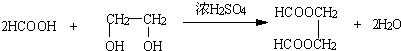 ��
��
�ʴ�Ϊ��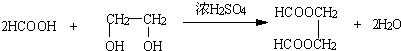 ��
��
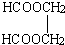 �������Ʊ�HCOOH��HOCH2CH2OH������CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO��HCHO������ΪHCOOH��CH3CHO����ԭΪCH3CH2OH��Ȼ��CH3CH2OH������ȥ��Ӧ����CH2=CH2��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br��ˮ�������HOCH2CH2OH�����߷���������Ӧ������
�������Ʊ�HCOOH��HOCH2CH2OH������CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO��HCHO������ΪHCOOH��CH3CHO����ԭΪCH3CH2OH��Ȼ��CH3CH2OH������ȥ��Ӧ����CH2=CH2��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br��ˮ�������HOCH2CH2OH�����߷���������Ӧ������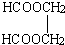 ��
����1��CH2CH=CH2Ϊԭ�ϣ�������HCHO��CH3CHO����Ӧ�ķ���ʽΪCH2CH=CH2
| O3 |
| Zn/H2O |
�ʴ�Ϊ��CH2CH=CH2
| O3 |
| Zn/H2O |
��2��HCHO������ΪHCOOH������ʽΪ2HCHO+O2
| ���� |
| ���� |
��3��CH3CHO����ԭΪCH3CH2OH������ʽΪCH3CHO+H2
| Ni |
| Ni |
��4��CH3CH2OH������ȥ��Ӧ����CH2=CH2������ʽΪCH3CH2OH
| Ũ���� |
| 170�� |
�ʴ�Ϊ��CH3CH2OH
| Ũ���� |
| 170�� |
��5��CH2=CH2�����ӳɷ�Ӧ����CH3BrCH2Br������ʽΪCH2=CH2��+Br2��CH3BrCH2Br��
�ʴ�Ϊ��CH2=CH2��+Br2��CH3BrCH2Br��
��6��CH3BrCH2Brˮ�������HOCH2CH2OH������ʽΪCH3BrCH2Br+2H2O
| NaOH |
�ʴ�Ϊ��CH3BrCH2Br+2H2O
| NaOH |
��7��HCOOH��HOCH2CH2OH����������Ӧ������
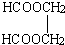 ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ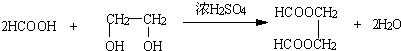 ��
���ʴ�Ϊ��
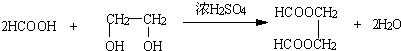 ��
�����������⿼���л���ĺϳɣ���Ŀ�Ѷ��еȣ�����ע�����������ƺϳ�·��Ϊ������Ĺؼ���ע���л�������ʣ�ѧϰ��ע����ۣ�

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�
�����Ŀ