��Ŀ����
��֪Ba��AlO2��2������ˮ����ͼ��ʾ������Al2��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�������ȷ���ǣ� ��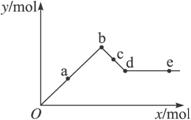
A.a��bʱ���������ʵ�����Al��OH��3��BaSO4��
B.c��dʱ��Һ�����ӵ����ʵ�����![]() ��Ba2+��
��Ba2+��
C.a��dʱ���������ʵ�����BaSO4����С��Al��OH��3
D.d��eʱ��Һ�����ӵ����ʵ�����Ba2+���ܵ���OH-
B
������a��b�ķ�ӦΪAl2��SO4��3+3Ba��OH��2====3BaSO4��+2Al��OH��3�������ֳ���ͬʱ���ɣ�n��BaSO4����n��Al��OH��3��=3��2��A�����c��d�ķ�ӦΪ2Al��OH3��+Ba��OH��2====Ba��AlO2��2+4H2O��Ba��AlO2��2���ܣ�n��![]() ��====2n��Ba2+����B����ȷ������������n��BaSO4��������n��Al��OH��3����C�����d��eʱ��Ba��OH��2������n��OH-��=2n��Ba2+����D�����
��====2n��Ba2+����B����ȷ������������n��BaSO4��������n��Al��OH��3����C�����d��eʱ��Ba��OH��2������n��OH-��=2n��Ba2+����D�����

��ϰ��ϵ�д�
�����Ŀ
��������CO2����ͨ��KOH��Ba��OH��2��KAlO2�Ļ����Һ�У����ɳ�����ͨ��CO2�����Ĺ�ϵ�ɱ�ʾΪ����֪Ba��AlO2�������ܣ���������
A��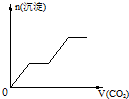 | B��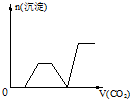 | C��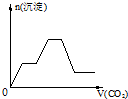 | D��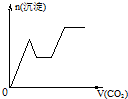 |
 ��֪Ba��AlO2��2������ˮ��ͼ��ʾ������Al2��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�������ȷ���ǣ�������
��֪Ba��AlO2��2������ˮ��ͼ��ʾ������Al2��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�������ȷ���ǣ�������| A��b��ʱ���������ʵ�����Al��OH��3��BaSO4 | B��c��ʱ��Һ�����ӵ����ʵ�����AlO2-��Ba2+ | C��a-bʱ���������ʵ�����BaSO4����С��Al��OH��3 | D��d-eʱ��Һ�����ӵ����ʵ�����Ba2+����OH- |
 ��֪Ba��AlO2��2������ˮ����ͼ��ʾ������A12��SO4��30.01mol����Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ������a��c�ֱ���0b�κ�bd�ε��е㣩�������й�������ȷ���ǣ�������
��֪Ba��AlO2��2������ˮ����ͼ��ʾ������A12��SO4��30.01mol����Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ������a��c�ֱ���0b�κ�bd�ε��е㣩�������й�������ȷ���ǣ������� ��2010?��ģ�⣩��֪Ba��AlO2��2������ˮ��������ͼ����100mL 0.02mol?L-1KAl��SO4��2��Һ����μ���0.05mol?L-1Ba��OH��2��Һʱ��25�棩�����ɳ��������ʵ��������Ba��OH��2��Һ������Ĺ�ϵ������˵������ȷ���ǣ�������
��2010?��ģ�⣩��֪Ba��AlO2��2������ˮ��������ͼ����100mL 0.02mol?L-1KAl��SO4��2��Һ����μ���0.05mol?L-1Ba��OH��2��Һʱ��25�棩�����ɳ��������ʵ��������Ba��OH��2��Һ������Ĺ�ϵ������˵������ȷ���ǣ������� ��֪Ba��AlO2��2������ˮ����ͼ��ʾ������Al2��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�����������ǣ�������
��֪Ba��AlO2��2������ˮ����ͼ��ʾ������Al2��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�����������ǣ�������