��Ŀ����
��1��Ũ�Ⱦ�Ϊ0.1mol/L��8����Һ����HNO3 ��H2SO4 ��CH3COOH ��Ba��OH��2 ��NaOH ��CH3COONa ��KCl ��NH4Cl����ҺpHֵ��С�����˳���ǣ�����д��ţ� ����2���Ȼ���ˮ��Һ�� �ԣ�ԭ���ǣ������ӷ���ʽ��ʾ���� ����AlCl3��Һ���ɣ����գ����õ�����Ҫ��������� ��
��3���ڢ�pH=3��������Һ�͢�pH=3�Ĵ�����Һ�У��ֱ�Ͷ������п�ۣ���ʼʱ��Ӧ���ʴ�СΪ�� �ڣ�����=������������ͬ������Ӧ���������ɵ�H2�������ͬ��ͬѹ���� �ڣ�����ˮϡ����ͬ�ı�����pHֵ�� �ڣ�
���𰸡���������1���Ƚ����ʵİ��ռ�Ρ����˳����࣬�ٸ����ε�ˮ���������ʵĵ����ص�Ƚ�PH��
��2���Ȼ���Ϊǿ�������Σ�����Al3+��ˮ���ص������
��3���Ӵ���Ϊ����ĽǶȷ�����
����⣺��1����Һ�ʼ��Ե��У��ܢݢޣ����Т�Ϊ��Ԫǿ���ΪһԪǿ���ˮ��ʼ��ԣ�Ũ����ͬʱ��PH���ܣ��ݣ��ޣ�
��Һ�����Ե��У��ߣ�
��Һ�����Ե��У��٢ڢۢ࣬���Т�ΪһԪǿ�ᣬ��Ϊ��Ԫǿ�ᣬ��Ϊ���ᣬ��ˮ������ԣ�Ũ����ͬʱ��
PH���ڣ��٣��ۣ��࣬
�ʴ�Ϊ���ڢ٢ۢ�ߢޢݢܣ�
��2���Ȼ���Ϊǿ�������Σ�Al3+��ˮ�⣺Al3++3H2O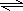 Al��OH��3+3H+����Һ�����ԣ�
Al��OH��3+3H+����Һ�����ԣ�
�Ȼ�����ˮ��Ϊ���ȹ��̣����ȴٽ�ˮ�⣬��AlCl3��Һ���ɣ��õ�Al��OH��3�����գ��ֽ�����Al2O3�����õ�����Ҫ���������Al2O3��
�ʴ�Ϊ���Al3++3H2O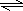 Al��OH��3+3H+��Al2O3��
Al��OH��3+3H+��Al2O3��
��3������Ϊ���ᣬpH=3��������Һ��pH=3�Ĵ�����Һ��Ƚϣ�����Ũ�ȴֱ�Ͷ������п�ۣ���ʼʱ������Һ��Ӧ���ʴ���������ʵ����࣬����ȫ��Ӧ���������࣬��ˮϡ��ʱ�������һ�����룬��ˮϡ����ͬ�ı���������PH�仯��Χ��
�ʴ�Ϊ��=����������
���������⿼������ˮ�⼰������ʵĵ��룬��Ŀ�Ѷ��еȣ�ע�������ˮ�⡢������ʵĵ����ص㣮
��2���Ȼ���Ϊǿ�������Σ�����Al3+��ˮ���ص������
��3���Ӵ���Ϊ����ĽǶȷ�����
����⣺��1����Һ�ʼ��Ե��У��ܢݢޣ����Т�Ϊ��Ԫǿ���ΪһԪǿ���ˮ��ʼ��ԣ�Ũ����ͬʱ��PH���ܣ��ݣ��ޣ�
��Һ�����Ե��У��ߣ�
��Һ�����Ե��У��٢ڢۢ࣬���Т�ΪһԪǿ�ᣬ��Ϊ��Ԫǿ�ᣬ��Ϊ���ᣬ��ˮ������ԣ�Ũ����ͬʱ��
PH���ڣ��٣��ۣ��࣬
�ʴ�Ϊ���ڢ٢ۢ�ߢޢݢܣ�
��2���Ȼ���Ϊǿ�������Σ�Al3+��ˮ�⣺Al3++3H2O
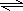 Al��OH��3+3H+����Һ�����ԣ�
Al��OH��3+3H+����Һ�����ԣ��Ȼ�����ˮ��Ϊ���ȹ��̣����ȴٽ�ˮ�⣬��AlCl3��Һ���ɣ��õ�Al��OH��3�����գ��ֽ�����Al2O3�����õ�����Ҫ���������Al2O3��
�ʴ�Ϊ���Al3++3H2O
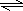 Al��OH��3+3H+��Al2O3��
Al��OH��3+3H+��Al2O3����3������Ϊ���ᣬpH=3��������Һ��pH=3�Ĵ�����Һ��Ƚϣ�����Ũ�ȴֱ�Ͷ������п�ۣ���ʼʱ������Һ��Ӧ���ʴ���������ʵ����࣬����ȫ��Ӧ���������࣬��ˮϡ��ʱ�������һ�����룬��ˮϡ����ͬ�ı���������PH�仯��Χ��
�ʴ�Ϊ��=����������
���������⿼������ˮ�⼰������ʵĵ��룬��Ŀ�Ѷ��еȣ�ע�������ˮ�⡢������ʵĵ����ص㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ