ћвƒњƒЏ»Ё
£®10Ј÷£ђ√њњ’2Ј÷£©ѕ÷”–Јі”¶£ЇҐўCO+CuO Cu+CO2 ҐЏMgO+2HCl====MgCl2+H2O
Cu+CO2 ҐЏMgO+2HCl====MgCl2+H2O
ҐџFe+2AgNO3====2Ag+Fe£®NO3£©2 Ґ№CH4+2O2 CO2+2H2O
CO2+2H2O
ҐЁNH4HCO3 NH3°ь+CO2°ь+H2O
NH3°ь+CO2°ь+H2O
‘Ќк≥…£Ї
£®1£©…ѕ цЈі”¶÷– ф”Џ÷√їїЈі”¶µƒ”–_____________°£ ф”ЏЄіЈ÷љвЈі”¶µƒ”–_____________°£
£®2£© ф”Џ—хїѓїє‘≠Јі”¶µƒ”–_____________°£
£®3£©Јі”¶Ґџ÷–±їїє‘≠µƒ‘™ЋЎ «_____________°£Јі”¶Ґ№÷–±ї—хїѓµƒ‘™ЋЎ «_____________°£
(10Ј÷£ђ√њњ’2Ј÷)£®1£©Ґџ ҐЏ £®2£©ҐўҐџҐ№ £®3£©“ш‘™ЋЎ ћЉ‘™ЋЎ
°Њљвќц°њ¬‘

£®10Ј÷£ђ√њњ’2Ј÷£©Њцґ®ќп÷ –‘÷ µƒ÷Ў“™“тЋЎ «ќп÷ µƒљбєє°£«лїЎірѕ¬Ѕ–ќ ћв£Ї
(1)“—÷™AЇЌBќ™µЏ»э÷№∆Џ‘™ЋЎ£ђ∆д‘≠„”µƒµЏ“ї÷ЅµЏЋƒµзјл»зѕ¬±нЋщ Њ£Ї
|
µзјлƒ№/kJ°§mol£≠1 |
I1 |
I2 |
I3 |
I4 |
|
A |
578 |
1 817 |
2 745 |
11 578 |
|
B |
738 |
1 451 |
7 733 |
10 540 |
AЌ®≥£ѕ‘____Љџ£ђAµƒµзЄЇ–‘__ __BµƒµзЄЇ–‘(ћо°∞£Њ°±°Ґ°∞£Љ°±їт°∞£љ°±)°£
(2)“—÷™£Ї≤®≥§ќ™300 nmµƒ„ѕЌвєвµƒєв„”ЋщЊя”–µƒƒ№Ѕњ‘Љќ™399 kJ°§mol£≠1°£ЄщЊЁѕ¬±н”–єЎµ∞∞„÷ Ј÷„”÷–÷Ў“™їѓ—ІЉьµƒ–≈ѕҐ£ђЋµ√ч»Ћће≥§ ±Љд’’…д„ѕЌвєвЇу∆§Јф“„ №…ЋЇ¶µƒ‘≠“т£Ї
°£
|
є≤ЉџЉь |
C°™C |
C°™N |
C°™S |
|
Љьƒ№/kJ°§mol£≠1 |
347 |
305 |
259 |
(3)—–Њњќп÷ і≈–‘±н√ч£Їљр ф—фјл„”Їђќі≥…ґ‘µз„”‘љґа£ђ‘ті≈–‘‘љіу£ђі≈Љ«¬Љ–‘ƒ№‘љЇ√°£јл„”–Ќ—хїѓќпV2O5ЇЌCrO2÷–£ђ Їѕ„ч¬Љ“фіші≈Јџ‘≠Ѕѕµƒ «________________°£
(4)ƒ≥≈дЇѕќпµƒЈ÷„”љбєє»зЌЉЋщ Њ£ђ∆дЈ÷„”ƒЏ≤їЇђ”–__________(ћо„÷ƒЄ)°£
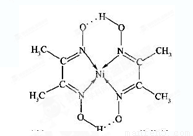
A£Ѓјл„”Љь B£Ѓє≤ЉџЉь
C£Ѓљр фЉь D£Ѓ≈дќїЉь E£Ѓ«вЉь
 C£®g£©+D£®g£©£ђ‘Џ≤їЌђќ¬ґ»ѕ¬£ђDµƒќп÷ µƒЅњn£®D£©ЇЌ ±ЉдtµƒєЎѕµ»зЌЉ°£ ‘їЎірѕ¬Ѕ–ќ ћв£Ї
C£®g£©+D£®g£©£ђ‘Џ≤їЌђќ¬ґ»ѕ¬£ђDµƒќп÷ µƒЅњn£®D£©ЇЌ ±ЉдtµƒєЎѕµ»зЌЉ°£ ‘їЎірѕ¬Ѕ–ќ ћв£Ї


