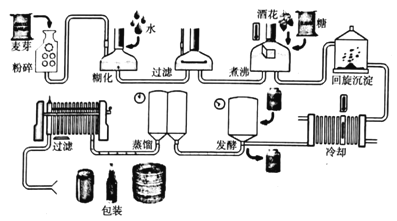
��Ŀ����
����Ŀ��һ���ݻ�ΪV���ܱ��������м���һ�����ɻ����ĸ���(��Ȳ���)�������ֳ������֣�����߳���1molN2���ұ߳���һ������COʱ�����崦����ͼλ��(�����¶Ȳ���)������˵����ȷ���ǣ� ��
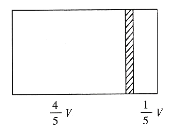
A.�ұ�����߷�����֮��Ϊ4��1
B.�Ҳ�CO������Ϊ5.6g
C.�Ҳ������ܶ�����ͬ�����������ܶȵ�14��
D.���ı��ұ�CO�ij�������ʹ���崦���������м䣬�����¶Ȳ��䣬��Ӧ����0.2molCO
���𰸡�C
��������
�������������¶ȡ�ѹǿ��ͬ����ͬ�����£����֮�ȵ������ʵ���֮�ȣ��������֮��Ϊ4��1���������������ʵ���֮��Ϊ4��1�������Ҳ��������ʵ���=![]() =0.25mol��
=0.25mol��
A��ͬ��ͬѹ�£��������������������ȣ����ұ�����߷�����֮��Ϊ1��4����A����
B���Ҳ�CO������Ϊ��28g/mol��0.25mol=7g����B����
C����ͬ�����£������ܶ�֮����Ħ�����������ȣ����Ҳ������ܶ�����ͬ�����������ܶȵ�![]() =14������C��ȷ��
=14������C��ȷ��
D��ͬ��ͬѹ�£�������������ʵ��������ȣ����崦���������м�ʱ������������������ʵ�����ȣ�����Ҫ����CO�����ʵ���Ϊ��1mol-0.25mol=0.75mol����D����
��ѡC��