��Ŀ����
����������Ԫ��A��B��C��D��E��F��ԭ�����������������ǵ�ԭ�Ӻ�����Ӳ���֮��Ϊ13����֪BԪ���γɵĻ�����������࣬DԪ��ԭ�������������Ǵ�����������3����Ԫ��E��ԭ�Ӱ뾶��ͬ���������FԪ��ԭ��������������������Ӳ�����
��1��CԪ����Ԫ�����ڱ��е�λ����
��2����A��B��D��E����Ԫ���γɵĻ������������Ļ�ѧ��������
��3��д��E2D2��A2D��Ӧ�����ӷ���ʽ
��4��ʵ��������100mL0.2mol?L-1��A��D��E�γɻ��������Һ����Ҫ�õ��IJ����������ձ���С�ձ����
��1��CԪ����Ԫ�����ڱ��е�λ����
�ڶ����ڵڢ�A��
�ڶ����ڵڢ�A��
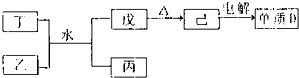
��B�ĵ�����C������������Ӧˮ�����Ũ��Һ������Ӧ�Ļ�ѧ����ʽ��C+4HNO3��Ũ��
CO2��+4NO2��+2H2O
| ||
C+4HNO3��Ũ��
CO2��+4NO2��+2H2O
| ||
��2����A��B��D��E����Ԫ���γɵĻ������������Ļ�ѧ��������
���Ӽ������ۼ�
���Ӽ������ۼ�
��3��д��E2D2��A2D��Ӧ�����ӷ���ʽ
2Na2O2+2H2O=4Na++4OH-+O2��
2Na2O2+2H2O=4Na++4OH-+O2��
����4��ʵ��������100mL0.2mol?L-1��A��D��E�γɻ��������Һ����Ҫ�õ��IJ����������ձ���С�ձ����
100mL����ƿ������������ͷ�ι�
100mL����ƿ������������ͷ�ι�
���������ʱ���ӿ̶��ߣ�����������ҺŨ����
��
0.2mol?L-1��������������������=��������F�ĵ��������������Ƶ���Һ����������Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=+2AlO2-+3H2��
2Al+2OH-+2H2O=+2AlO2-+3H2��
������������������Ԫ��A��B��C��D��E��F��ԭ�����������������ǵ�ԭ�Ӻ�����Ӳ���֮��Ϊ13����֪BԪ���γɵĻ�����������࣬��BΪ̼Ԫ�أ�DԪ��ԭ�������������Ǵ�����������3������Dԭ����2�����Ӳ㣬����������Ϊ6����DΪ��Ԫ�أ�C��ԭ����������̼Ԫ������Ԫ��֮�䣬��CΪ��Ԫ�أ�Ԫ��E��ԭ�Ӱ뾶��ͬ���������ԭ������������Ԫ�أ��ʴ��ڵ������ڣ�EΪNaԪ�أ�FԪ��ԭ��������������������Ӳ�����ԭ����������NaԪ�أ����ڵ������ڣ���FΪAlԪ�أ�AԪ��ԭ�ӵ��Ӳ���Ϊ13-2-2-2-3-3=1����AΪ��Ԫ�أ��ݴ˽��
����⣺����������Ԫ��A��B��C��D��E��F��ԭ�����������������ǵ�ԭ�Ӻ�����Ӳ���֮��Ϊ13����֪BԪ���γɵĻ�����������࣬��BΪ̼Ԫ�أ�DԪ��ԭ�������������Ǵ�����������3������Dԭ����2�����Ӳ㣬����������Ϊ6����DΪ��Ԫ�أ�C��ԭ����������̼Ԫ������Ԫ��֮�䣬��CΪ��Ԫ�أ�Ԫ��E��ԭ�Ӱ뾶��ͬ���������ԭ������������Ԫ�أ��ʴ��ڵ������ڣ�EΪNaԪ�أ�FԪ��ԭ��������������������Ӳ�����ԭ����������NaԪ�أ����ڵ������ڣ���FΪAlԪ�أ�AԪ��ԭ�ӵ��Ӳ���Ϊ13-2-2-2-3-3=1����AΪ��Ԫ�أ�
��1��CΪ��Ԫ�أ���Ԫ�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬̼������Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽ��C+4HNO3��Ũ��
CO2��+4NO2��+2H2O��
�ʴ�Ϊ���ڶ����ڵڢ�A�壻C+4HNO3��Ũ��
CO2��+4NO2��+2H2O��
��2����H��C��O��Na����Ԫ���γɵĻ�����Ϊ̼�����ơ������Ƶȣ������Ļ�ѧ�����������Ӽ������ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
��3��Na2O2��H2O��Ӧ�����ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH-+O2�����ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH-+O2����
��4��ʵ��������100mL0.2mol?L-1��NaOH��Һ����Ҫ�õ��IJ����������ձ���С�ձ���У�100mL����ƿ������������ͷ�ιܣ��������ʱ���ӿ̶��ߣ�Һ���ڿ̶����Ϸ�����Һ���ƫ������������ҺŨ�ȣ�0.2mol?L-1����Al�ĵ���������������NaOH����Һ����������Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=+2AlO2-+3H2����
�ʴ�Ϊ��100mL����ƿ������������ͷ�ιܣ�����2Al+2OH-+2H2O=+2AlO2-+3H2����
��1��CΪ��Ԫ�أ���Ԫ�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬̼������Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽ��C+4HNO3��Ũ��
| ||
�ʴ�Ϊ���ڶ����ڵڢ�A�壻C+4HNO3��Ũ��
| ||
��2����H��C��O��Na����Ԫ���γɵĻ�����Ϊ̼�����ơ������Ƶȣ������Ļ�ѧ�����������Ӽ������ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
��3��Na2O2��H2O��Ӧ�����ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH-+O2�����ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH-+O2����
��4��ʵ��������100mL0.2mol?L-1��NaOH��Һ����Ҫ�õ��IJ����������ձ���С�ձ���У�100mL����ƿ������������ͷ�ιܣ��������ʱ���ӿ̶��ߣ�Һ���ڿ̶����Ϸ�����Һ���ƫ������������ҺŨ�ȣ�0.2mol?L-1����Al�ĵ���������������NaOH����Һ����������Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=+2AlO2-+3H2����
�ʴ�Ϊ��100mL����ƿ������������ͷ�ιܣ�����2Al+2OH-+2H2O=+2AlO2-+3H2����
���������⿼��ṹ����λ�ù�ϵ��Ԫ�ػ��������ʡ����û�ѧ�����Һ���Ƶȣ��Ѷ��еȣ��ƶ�Ԫ���ǹؼ������ضԻ���֪ʶ�Ĺ��̣�

��ϰ��ϵ�д�
�����Ŀ
����������Ԫ��A��B��C��D��ԭ��������������A��Cԭ���������8��A��B��C����Ԫ��ԭ�ӵ�����������֮��Ϊ15��Bԭ����������������Aԭ��������������һ�룮����������ȷ���ǣ�������
| A��ԭ�Ӱ뾶��A��D��C��B | B��B��C��D�ֱ���A�γɵĻ�����һ��������ͬ�Ļ�ѧ�� | C������������Ӧˮ��������ԣ�D��C | D�������£�����B�ܴ�������Ũ������ |
����������Ԫ��A��B��C��ԭ���������ε�����A��Cͬ���壬Bԭ�ӵ���������������Aԭ�ӵĴ���������������ԭ�ӵ�����������֮��Ϊ10��������������ȷ���ǣ�������
| A��ԭ�ڰ뾶A��B��C | B��A����̬�⻯���ȶ��Դ���C����̬�⻯���ȶ��� | C��A��C��Ԫ����������������ˮ���ϵõ���Ӧ���� | D������ʱ��A���ʿ��Դ�C�����������û��õ�C���� |
����������Ԫ��A��B��C��D��E��ԭ������������������A��Cͬ���壬A������Ԫ�ز���ͬһ���ڣ�B��Dͬ���壬������D�ĵ���Ϊ����ɫ���壮�����ƶ�����ȷ���ǣ�������
| A��ԭ�Ӱ뾶��С�����˳��r��C����r��D����r��E�� | B��Ԫ��D��E�ֱ���A�γɵĻ���������ȶ��ԣ�E��D | C��Ԫ��D������������Ӧˮ��������Ա�E��ǿ | D��Ԫ��B�ֱ���A��C�γɵĻ������л�ѧ����������ȫ��ͬ |
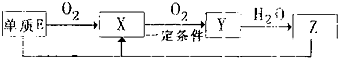
 ����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ����
����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ���� NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2