��Ŀ����
��֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2��(1)��Ӧ�м�����Ҵ��ǹ����ģ���Ŀ����_____________��
(2)�ߵμӴ��ᣬ���������Ŀ����_____________��
���ֲ�Ʒ�پ����в��辫�ƣ�
(3)Ϊ��ȥ���еĴ��ᣬ�����Ʒ�м���___________(����ĸ)��
A.��ˮ�Ҵ� B.̼���Ʒ�ĩ C.��ˮ������
(4)�������м��뱥���Ȼ�����Һ�������룬��Ŀ����_____________��
(5)Ȼ���������м�����ˮ�����ƣ�����Ŀ����_____________��
������������������Һ�������һ�����������ƿ�ڣ���������ȥ�ͷе���֣��ռ��е���76�桪
�����������ᷢ��������Ӧ�ǿ���ģ����ɵ�������ˮ�����ɴ����ᡣ
CH3COOH+CH3CH2OH![]() C2H5OOCCH3+H2O
C2H5OOCCH3+H2O
(����) (���)
Ϊ��ʹ��Ӧ���ϵ����������������ķ�����У���ȡ��ʹ�ù����Ҵ��Ͳ����������������Ĵ�ʩ�����ڷ�Ӧ��������ķе�ȽϽӽ���������ʱ�ɴ��������Ļ�����õ��������������и����������ʣ������Ҫ���ơ����ƹ�����ѡ�õĸ������ʼ�����Ӧ����Ҫ��ȥCH3COOH��CH3CH2OH��H2O��
�𰸣�(1)����Ӧ���Ҵ���Ũ�ȣ������ڷ�Ӧ���������������ķ������
(2)���Ӵ���Ũ�ȣ���С����������������Ũ�ȣ�������������Ӧ���������������ķ������
(3)B (4)��ȥ�ֲ�Ʒ�е��Ҵ� (5)��ȥ�ֲ�Ʒ�е�ˮ

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�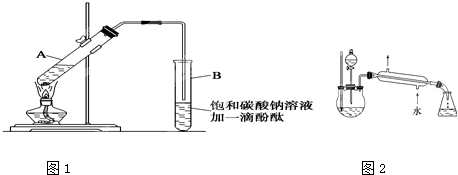
 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O