��Ŀ����
���л��ﻯѧ������
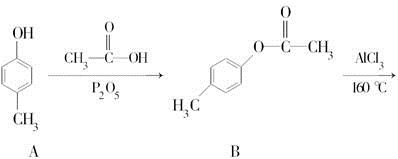
ƻ����㷺������ƻ����ˮ���Ĺ����У���һ�ֳ��õ�ʳƷ���Ӽ������ⶨ��ƻ�������Է�������Ϊ134��������Ԫ�ص���������Ϊ��w(c)="35.82%" W(H)=4.86%,����Ϊ�������д���5�ֲ�ͬ��ѧ������Hԭ�ӡ�1molƻ��������2molNaHCO3��ȫ��Ӧ������������Na��Ӧ����1.5molH2�ġ�����ϩΪԭ���˹��ϳ�ƻ�������·���£�

��֪��
��ش��������⣺
��1��ƻ����ķ���ʽΪ_______��A���ʵ�����Ϊ_______��
��2��F�к��еĹ�����������_______��G+B��H�ķ�Ӧ������_______��
��3���ںϳ���·�У�C��D��һ���跴Ӧ��Ŀ����_____��
��4��D��E��Ӧ�Ļ�ѧ����ʽΪ_________��
��5��ƻ�����NaHCO3��ȫ��Ӧ�Ļ�ѧ����ʽΪ________��
��6����ƻ���Ậ����ͬ����������Ĺ����ŵ�ͬ���칹��Ľṹ��ʽΪ____��
��1��C4H6O5 �Ҵ�
��2�� �ǻ����Ȼ� �ӳɷ�Ӧ��
��3����C����������Brԭ�ӣ�
��4��Br��CH2��COOH+2NaOH HO��CH2��COONa+NaBr+H2O
HO��CH2��COONa+NaBr+H2O
��5��
��6��
����

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д��Լ�����������( )�����ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
)�����ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
(1)D�к��й����ŵ�������________��A��E�ķ�Ӧ����Ϊ______��
(2)G�Ľṹ��ʽΪ_______��
(3)д��1�����������ұ�����ֻ��һ��ȡ������C8H8O2��ͬ���칹�� ��
(4)�����( )�ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________(�����)��
)�ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________(�����)��
| A��NaOH��Һ | B��NaHCO3��Һ | C��KMnO4/H+ | D��FeCl3��Һ |


 �����
�����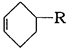 ��
��



 )��һ����Ҫ�Ļ���ԭ�ϡ���д���Ա�����ȩ���Ҵ�Ϊ��Ҫԭ���Ʊ������������ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
)��һ����Ҫ�Ļ���ԭ�ϡ���д���Ա�����ȩ���Ҵ�Ϊ��Ҫԭ���Ʊ������������ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�

 (1)�١���������ȡ����Ӧ���� ��
(1)�١���������ȡ����Ӧ���� ��
 ͨ������·�߿ɺϳ�(��)��
ͨ������·�߿ɺϳ�(��)��
 �����ɣ���ʱ�����ܵõ���һ�ָ�����C6H8O4���÷�Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�ķ�Ӧ������ ��
�����ɣ���ʱ�����ܵõ���һ�ָ�����C6H8O4���÷�Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�ķ�Ӧ������ �� Ҳ�����л���(��) (����)�������Ʒ�Ӧ�ٵ�ϵ�з�Ӧ���������л���Ľṹ��ʽΪ ��
Ҳ�����л���(��) (����)�������Ʒ�Ӧ�ٵ�ϵ�з�Ӧ���������л���Ľṹ��ʽΪ ��