��Ŀ����
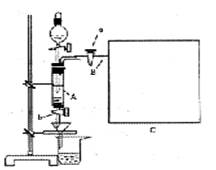
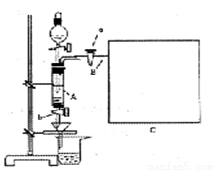
���Ȼ�������ѧ��ѧʵ�����бز����ٵ���Ҫ�Լ���ijͬѧ���÷���м��������ͭ�Ȳ������ᷴӦ�����ʣ����Ʊ�FeCl3��6H2O����ͬѧ��Ƶ�ʵ��װ������ͼ��ʾ��A�з���m�˷���м���ձ���ʢ�й�����ϡ���ᣬʵ��ʱ��a���ر�b���ӷ�Һ©������A�мӹ�����ϡ���ᣬ��ʱ��Һ��dz��ɫ���ٴ�b���й��ˣ����˽�����ȡ�ձ�����Һ������������ȣ�����������ˮ��ʹ����HNO3�ֽ⣬�ٽ��½ᾧ��FeCl3��6H2O���塣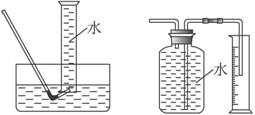
������������⣺
��1����μ��װ��A�������ԣ�___________________________________________________��
��2���μ�����ʱ�����ַ�Ӧ���ʽ�֮ͬŨ�������봿���۷�ӦҪ�죬��ԭ����____________________________________________________________________��
��3�����ձ�����Һ��������Ũ�����ٽ��½ᾧ���Ƶ�FeCl3��6H2O���壬������ֱ�������ᾧ�ķ������Ƶþ����������___________________________________________________��
��4���ø÷��Ƶõľ�������������Fe��NO3��3��Ϊ���Ƶýϴ�����FeCl3��6H2O���ɽ��ձ��ڵ�ϡ���ỻ��_____________________________________________________________��
��5����Ҫ�����м�Ĵ��ȣ��ɲ����B�зų���������V��������ɱ������λ��L���������м�Ĵ���Ϊ��____________����m��V�Ĵ���ʽ��ʾ�������ڿ�ͼC���л�����Ҫ��װ�á�
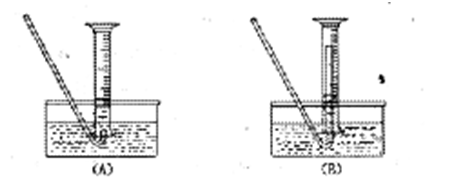
��1���ٹرջ���a������b����Һ©���������϶����ӣ�����עˮ��©���¶˻��γ�һ��Һ����һ��ʱ�����Һ���������ƣ�˵�������Բ��ã���Һ��ͣס��������˵�����������á��ڹرշ�Һ©�������ͻ���b������a�����ܿ�B��һ�δ������ܵ��齺�ܲ�����ˮ��ˮ�У�˫����סװ��A����ˮ���������ݲ������ſ�˫�ֲ��������γ�һ��Һ����������������
��2������������ͭ�γ�ԭ��ط�Ӧ���ӿ��˷�Ӧ����
��3����ΪFeCl3��ǿ�����������Իᷢ�����µ�ˮ�ⷴӦ��FeCl3+3H2O![]() Fe��OH��3+3HCl����������ʱʹHCl�ӷ����ˮ��ƽ�����ƣ���˵ò���FeCl3��6H2O
Fe��OH��3+3HCl����������ʱʹHCl�ӷ����ˮ��ƽ�����ƣ���˵ò���FeCl3��6H2O
��4��˫��ˮ��������ˮ
��5��![]() ��
��![]()