��Ŀ����
����Ŀ��������Դ�����þ��й���ǰ����
(1)���в����ں�ˮ�����ķ���________(�����)��
A������ B�����ӽ�����
C�����˷� D����������
(2)��ͼ�ǴӺ�ˮ����ȡþ�ļ����̡�
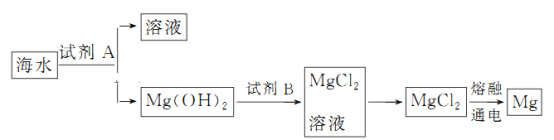
���Լ�B��________(�ѧʽ)��
������ˮMgCl2��ȡMg�Ļ�ѧ����ʽ��__________________________________________
(3)�������и�����I����ʽ���ڵĵ�Ԫ�ء�ʵ������ȡI2��;��������ʾ��
����![]() ������
������![]() ����������Һ
����������Һ![]() ��I����Һ
��I����Һ![]() ��I2ˮ��Һ
��I2ˮ��Һ![]() ��I2����Һ
��I2����Һ![]() ����I2
����I2
��.���в����ڲ������������õ�������________(�����)��
A���ƾ��� B��©��
C������ D��������
��.��������ʵ�����������________��
��.�ܲ��跴Ӧ�����ӷ���ʽ____________________________________________________
���𰸡�C HCl MgCl2(����)![]() Mg��Cl2�� B ���� H2O2��2H����2I��=I2��2H2O
Mg��Cl2�� B ���� H2O2��2H����2I��=I2��2H2O
��������
��ȡMg�����̣���ˮ�м��Լ�AΪ��ʯ�ң���þ����ת��Ϊ�������Լ�BΪ���ᣬ����������Ȼ�þ�õ�Mg��ʵ������ȡI2��;�������������������գ��ܽ����˵õ���I-����Һ���ӹ������������õ����ⵥ�ʵ���Һ������ȡ����õ��⡣
��1��A�������ǰ�ˮ��ˮ�Ļ�����з���������õ�������ˮ����A��ѡ��B��ͨ�����ӽ�����֬���Գ�ȥ��ˮ�е����ӣ��Ӷ��ﵽ������ˮ��Ŀ�ģ���B��ѡ��C������ֻ�ܳ�ȥˮ�еIJ��������ʣ����ܵ�����ˮ����Cѡ��D�����õ���������ʹ��Ӧ������ͨ����Ĥ�Դﵽ������ˮ��Ч������D��ѡ��
����C��
��2������ȡMg�����̿�֪����ˮ�м��Լ�AΪ��ʯ�ң���þ����ת��Ϊ�������Լ�BΪ���ᣬ����������Ȼ�þ�õ�Mg����������������֪��BΪHCl��������ˮMgCl2��ȡMg�Ļ�ѧ����ʽ��MgCl2(����)![]() Mg��Cl2����
Mg��Cl2����
��3����ʵ������ȡI2��;����֪�����������������գ��ܽ����˵õ���I-����Һ���ӹ������������õ����ⵥ�ʵ���Һ����Ϊ��ȡ����Ϊ����õ��⣻
I������ٲ�����Ҫ�ƾ��ơ������������ǣ�����Ҫ©�����ʴ�Ϊ��B��
II��������������֪������۵�ʵ����������ǹ��ˣ�
�ܲ��跢��������ԭ��Ӧ����Ӧ�����ӷ���ʽΪH2O2+2H++2I-=I2+2H2O��

����Ŀ�������ǻ����л�����ԭ��֮һ���㷺����ũҩ��Ƥ�Ⱦ�ϡ�ҽҩ���ȹ�ҵ��
(1)��ҵ�����ü����������ϵת��ͼ��ͼ��

��Ӧ![]() ���ʱ�
���ʱ�![]() ________
________![]() ��
��
(2)ij��ѧС���о���ͬѹǿ�����Է�Ӧ![]() ��Ӱ�졣
��Ӱ�졣![]() �£���һ���ݻ��ɱ���ܱ������У�����һ������
�£���һ���ݻ��ɱ���ܱ������У�����һ������![]() ��
��![]() ����ò�ͬѹǿ�£�ƽ��ʱ�����������Ũ�����±���
����ò�ͬѹǿ�£�ƽ��ʱ�����������Ũ�����±���
������ | ��Ӧѹǿ | ����Ũ�� | ||
|
|
| ||
1 |
| 0.3 | 0.3 | 0.9 |
2 |
|
|
| 0.4 |
3 |
| 0.4 | 0.4 |
|
�Իش��������⣺
��ƽ��ʱ��ʵ��1������Ӧ����________(����>������<������=��)ʵ��3���淴Ӧ���ʡ�
����ʵ��1�����ݿɼ���![]() ʱ���÷�Ӧ��ƽ�ⳣ��
ʱ���÷�Ӧ��ƽ�ⳣ��![]() ________��
________��
��![]() ________
________![]() ��
��
(3)���о����ֲ��õ绹ԭ��Ҳ�ɽ�![]() ת��Ϊ�������ͬʱ�������
ת��Ϊ�������ͬʱ�������![]() ��ת��Ч�ʡ�����ԭ����ͼ��ʾ������������ȷ����________��
��ת��Ч�ʡ�����ԭ����ͼ��ʾ������������ȷ����________��
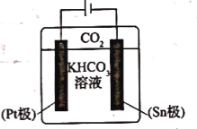
A.![]() ���ĵ缫��ӦʽΪ
���ĵ缫��ӦʽΪ![]()
B.��������![]() ��
��![]() ���ƶ�
���ƶ�
C.![]() ��������ԭ��Ӧ���������ݳ�
��������ԭ��Ӧ���������ݳ�
D.��������![]() Ũ����С
Ũ����С
(4)�������;֮һ������������������Һ������������Һ�м���������ǿ��ʴ���Һ��![]() �仯�����ܱ�����Һ
�仯�����ܱ�����Һ![]() ����ȶ���(��֪����ĵ���ƽ�ⳣ��
����ȶ���(��֪����ĵ���ƽ�ⳣ��![]() )
)
���ֽ���Ũ�ȼ������������Һ��ϣ����![]() ��
��![]() ������Һ�������ӷ���ʽ��ʾ������ǿ�����
������Һ�������ӷ���ʽ��ʾ������ǿ�����![]() ������Һ�У�
������Һ�У�![]() �仯�����ԭ����________��
�仯�����ԭ����________��
������![]() ��Һ����
��Һ����![]() Ϊ4�Ļ�����Һ�������________
Ϊ4�Ļ�����Һ�������________![]() (�𰸱���һλС��)
(�𰸱���һλС��)![]() ��Һ��
��Һ��
����Ŀ��ʵ������NaOH��������250 mL 1.25 mol/L��NaOH��Һ,��ղ���ش���������:
��1������250 mL 1.25 mol/L��NaOH��Һ
Ӧ��ȡNaOH������/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ�������������� |
__________ | __________ | __________ |
��2������ƿ����������������е�__________;
���¶� ��Ũ�� ������ ��ѹǿ �ݿ̶���
��3������ʱ,����ȷ�IJ���˳����(��ĸ��ʾ,ÿ����ĸֻ����һ��)__________;
A����30 mLˮϴ���ձ�2��3��,ϴ��Һ��ע������ƿ,��
B������ƽȷ��ȡ�����NaOH������,��������ˮ(Լ30 mL),�ò�������������,ʹ�����ܽ�
C��������ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
D��������ƿ�ǽ�,�ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ,ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ,ֱ��Һ��ӽ��̶�1��2cm��
��4������A��,��ϴ��Һ����������ƿ,��Ŀ����__________,��Һע������ƿǰ��ָ�������,������Ϊ__________
��5���������Ƶ���ҺŨ��ƫ�͵�����_____��
A.����NaOHʱ,�������������
B.������ƿ��ת����Һʱ(ʵ�鲽���)������Һ����������ƿ����
C.������ˮʱ���������˿̶���
D.����ʱ���ӿ̶���
E.����ǰ,����ƿ������������ˮ



