��Ŀ����
��֪�������µ��ܶȻ�����Ksp��BaCO3��=2.6��10-6��Ksp=��BaSO4��=1.1��10-7����֪��ʹBaSO4ת��ΪBaCO3�������ǽ�BaSO4����NaCO3��Һ�в����Ͻ��裮
��1��д������ת�������ӷ���ʽ�� �÷�Ӧ��ƽ�ⳣ������Ϊ ��
��2����1.0LNa2CO3��Һ��0.010mol��BaSO4��ȫת������Na2CO3��Һ�����Ũ��Ӧ������ ��
��1��д������ת�������ӷ���ʽ��
��2����1.0LNa2CO3��Һ��0.010mol��BaSO4��ȫת������Na2CO3��Һ�����Ũ��Ӧ������
���㣺���ܵ���ʵ��ܽ�ƽ�⼰����ת���ı���
ר�⣺����ƽ������Һ��pHר��
��������1��BaSO4ת��ΪBaCO3��BaSO4 ��s��+CO32- ��aq��?BaCO3 ��s��+SO42- ��aq��������K=
=
���㣻
��2��Qc=c��SO42-��?c��Ba2+����Ksp��BaSO4 ����BaSO4��ȫ�ܽ⣬�ݴ˼��㣻
| c(SO42-) |
| c(CO32-) |
| Ksp(BaSO4) |
| Ksp(BaCO3) |
��2��Qc=c��SO42-��?c��Ba2+����Ksp��BaSO4 ����BaSO4��ȫ�ܽ⣬�ݴ˼��㣻
���
�⣺��1�������µ��ܶȻ�����Ksp��BaCO3��=2.6��10-6��Ksp=��BaSO4��=1.1��10-7����c��CO32-���㹻��ʱ��������Qc=c��CO32-��?c��Ba2+����Ksp��BaCO3����BaSO4��ת��ΪBaCO3�������ǽ�BaSO4����NaCO3��Һ�в����Ͻ��裬��ӦΪBaSO4 ��s��+CO32- ��aq��?BaCO3 ��s��+SO42- ��aq����K=
=
=
=0.042��
�ʴ�Ϊ��0.042��
��2��CO32-+BaSO4=BaCO3+SO42-��K=
=
=
=0.042��
c��SO42-��=0.01 mol������
��0.042��c��CO32-����0.238+0.01=0.338 mol/L��
�ʴ�Ϊ��0.338 mol/L��
| c(SO42-) |
| c(CO32-) |
| Ksp(BaSO4) |
| Ksp(BaCO3) |
| 1.1��10-7 |
| 2.6��10-6 |
�ʴ�Ϊ��0.042��
��2��CO32-+BaSO4=BaCO3+SO42-��K=
| c(SO42-) |
| c(CO32-) |
| Ksp(BaSO4) |
| Ksp(BaCO3) |
| 1.1��10-7 |
| 2.6��10-6 |
c��SO42-��=0.01 mol������
| 0.01mol |
| c(CO32-) |
�ʴ�Ϊ��0.338 mol/L��
���������⿼���˳���֮���ת���������ܽ�ƽ����ƶ���Ksp���йؼ��㣬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�ע����ճ����ܽ�ƽ���ԭ���ǽ���Ĺؼ���

��ϰ��ϵ�д�
 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
���л�ѧ��Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A����С�մ�����θ����ࣺHCO3-+H+=CO2��+H2O |
| B����̼����еμ�ϡ���CO32-+2H+=CO2��+H2O |
| C������ˮ�еμ��Ȼ�����Һ��Al3++3OH-=Al��OH��3 |
| D������������Һ��ϡ���ᷴӦ��Ba2++SO42-+H++ OH-=BaSO4��+H2O |
���и���Ӧ�����ڼӳɷ�Ӧ���ǣ�������
A��CH2�TCH2+H-OH
| |||
B��H2+Cl2
| |||
C��2CH3-CH2-OH+O2
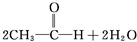 | |||
D��CH3-CH3+2Cl2
|
4-�廷��ϩ�� ����������4�����ʷ�����Ӧ����HBr�������Ը��������Һ���������Ƶ�ˮ��Һ�����������Ƶ��Ҵ���Һ�������й���Щ��Ӧ��˵������ȷ���ǣ�������
����������4�����ʷ�����Ӧ����HBr�������Ը��������Һ���������Ƶ�ˮ��Һ�����������Ƶ��Ҵ���Һ�������й���Щ��Ӧ��˵������ȷ���ǣ�������
 ����������4�����ʷ�����Ӧ����HBr�������Ը��������Һ���������Ƶ�ˮ��Һ�����������Ƶ��Ҵ���Һ�������й���Щ��Ӧ��˵������ȷ���ǣ�������
����������4�����ʷ�����Ӧ����HBr�������Ը��������Һ���������Ƶ�ˮ��Һ�����������Ƶ��Ҵ���Һ�������й���Щ��Ӧ��˵������ȷ���ǣ�������| A����ܷ�Ӧ��õ����л�����ֻ��һ�ֹ����� |
| B����۵ķ�Ӧ������ȥ��Ӧ |
| C����ڵķ�Ӧ������ϩ��ʹ��ˮ��ɫ |
| D����ٷ�Ӧ����л�����ֻ��һ�� |
ʵ������2mol?L-1��Na2CO3��Һ950mL������ʱӦѡ�õ�����ƿ�Ĺ��ͳ�ȡNa2CO3�������ǣ�������
| A��1000 mL��212 g |
| B��950 mL��543.4 g |
| C��������572 g |
| D��500 mL��286 g |
����˵��������ǣ�������
| A��ԭ������������Ϊ2��Ԫ��һ������Ԫ�����ڱ��ڢ�A�� |
| B��L�������Ϊ����������Ԫ����������������Ԫ��ԭ�ӵ�L���������ͬ |
| C������������Ԫ��X��Y���γ�XY2�ͻ������X��Y ��ԭ������֮�����Ϊ1��2 |
| D��M�������Ϊ��������������Ԫ����������������Ԫ��ԭ�ӵ�M���������ͬ |

 b����ϡ������ˮ������������
b����ϡ������ˮ������������

 ����V�����仯����㷺Ӧ���ڹ�ҵ�����²��Ϻ�����Դ������
����V�����仯����㷺Ӧ���ڹ�ҵ�����²��Ϻ�����Դ������