��Ŀ����
 ij��ѧ�о�С��ͨ�������������Ϸ���N2����ȡ���������ַ�����
ij��ѧ�о�С��ͨ�������������Ϸ���N2����ȡ���������ַ�����
����a����������������NH3��ԭCuO�Ƶô�����N2�ͻ���ͭ�ۣ�
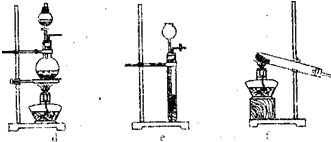
����b������������ͨ�����ȵ�ͭ���Ƶýϴ�����N2����ʵ�����ṩ���¼���װ������ȡN2��
��1����������a��ȡN2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲�������װ��______������ţ���Ϊ���������÷���װ���з�����Ӧ�Ļ�ѧ����ʽΪ______��Ҫ��ȡ���ռ�������N2��������������ˮ������������ʹ�õ�����װ�ã������������ҵ�����˳���г���______������ţ���
��2����������b��ȡN2����װ��E����������ͨ��______���A������B����C������Ӧ�����÷�Ӧ���ڿɹ۲쵽��������______���÷�Ӧ�Ļ�ѧ����ʽΪ______��
�⣺��1��Ũ��ˮ�ӷ���CaO������ˮ��Ӧ�ų��������ȣ������¶����ߣ�ʹ�ð�����ˮ�е��ܽ�Ƚ�һ�����٣����������ʽ�ݳ����Ƶð���������������װ�ã��������Ʊ�����ķ���װ�õ���BE��Eװ�õ��ŵ����ܿ��Ʒ�Ӧ�����ʣ���Bװ��ֻ��һ���Լ���ҩƷ�������Ʒ�Ӧ�����ʣ�����ѡ��ͼ�е�Eװ�ã������Ļ�ѧ��ӦΪ��NH3?H2O+CaO�TCa��OH��2+NH3����������a��ȡN2�лẬ��δ��Ӧ�İ�������ˣ�����Ҫ���Լ����հ��������ڰ�����һ�ּ�������ˮ�����壬��������ˮ���ռ����������ɳ�ȥ�����к��еİ��������Ի���ʹ�õ�װ����ACD��
�ʴ�Ϊ��E��NH3?H2O+CaO�TCa��OH��2+NH3����ACD��
��2��������Ҫ���������͵������ɣ����÷�Һ©���е�ˮ�ɽ���������ƿ���ŵ�װ��A�У��ڼ��ȵ�������ͭ�Ϳ����е�������Ӧ���Ӷ���ȥ�����е�����������ʣ�൪�������Ƶýϴ�����N2������ͭΪ��ɫ��ͭΪ��ɫ�����Կɹ۲쵽�������Ǻ�ɫ��ĩ��ڣ��÷�Ӧ�Ļ�ѧ����ʽΪO2+2Cu CuO��
CuO��
�ʴ�Ϊ��A����ɫ��ĩ��ڣ�O2+2Cu CuO��
CuO��
��������1������ʯ�Һ�Ũ��ˮ��ԭ����ȡ��������ѡ������Һ�岻��Ҫ������ȡ�����װ�ã��ṩ��װ��BE������������Bֻ��һ���Լ���ҩƷ�������Ʒ�Ӧ�����ʣ�����ѡ��E�������ṩ�ķ�Ӧ����д��ѧ����ʽ��������ȡ���ռ�������N2��ԭ��ѡ��װ�ã�
��2��������Ҫ���������͵������ɣ�����������ͨ�����ȵ�ͭ�ۣ�ͭ�ۺ�������Ӧ��������ʣ�൪�������Կ��Ƶýϴ�����N2����װ��E����������ͨ��A���ٸ���������н����д��ѧ����ʽ��
������������Ҫ������ʵ�����Ƶ��������տ�������ɡ�ͭ���仯����������ǽ����Ĺؼ�����Ŀ�ѶȲ���
�ʴ�Ϊ��E��NH3?H2O+CaO�TCa��OH��2+NH3����ACD��
��2��������Ҫ���������͵������ɣ����÷�Һ©���е�ˮ�ɽ���������ƿ���ŵ�װ��A�У��ڼ��ȵ�������ͭ�Ϳ����е�������Ӧ���Ӷ���ȥ�����е�����������ʣ�൪�������Ƶýϴ�����N2������ͭΪ��ɫ��ͭΪ��ɫ�����Կɹ۲쵽�������Ǻ�ɫ��ĩ��ڣ��÷�Ӧ�Ļ�ѧ����ʽΪO2+2Cu
 CuO��
CuO���ʴ�Ϊ��A����ɫ��ĩ��ڣ�O2+2Cu
 CuO��
CuO����������1������ʯ�Һ�Ũ��ˮ��ԭ����ȡ��������ѡ������Һ�岻��Ҫ������ȡ�����װ�ã��ṩ��װ��BE������������Bֻ��һ���Լ���ҩƷ�������Ʒ�Ӧ�����ʣ�����ѡ��E�������ṩ�ķ�Ӧ����д��ѧ����ʽ��������ȡ���ռ�������N2��ԭ��ѡ��װ�ã�
��2��������Ҫ���������͵������ɣ�����������ͨ�����ȵ�ͭ�ۣ�ͭ�ۺ�������Ӧ��������ʣ�൪�������Կ��Ƶýϴ�����N2����װ��E����������ͨ��A���ٸ���������н����д��ѧ����ʽ��
������������Ҫ������ʵ�����Ƶ��������տ�������ɡ�ͭ���仯����������ǽ����Ĺؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ


 ������õ��˸ߴ��ȵ��������ۣ�
������õ��˸ߴ��ȵ��������ۣ�




