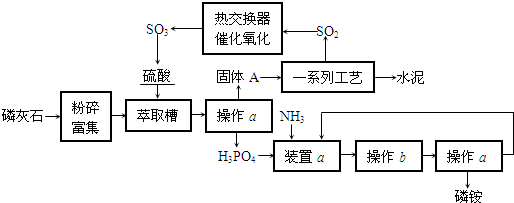
��Ŀ����
������ҹ�������������ŷŵķ�����ʯ����ȡ���Ტ����ˮ��ļ����о���óɹ������������������£�

�ش��������⣺
��1��������a������b����ʵ���ҽ��У������aʱ�õ��IJ���������______�����в���bʱ��ע��______��
��2��װ��a����������NH3�����ù�����ʵ�����н��У��뻭��װ��a��ʾ��ͼ��
��3���Ƚ�������ʵ�����Ƚ�����װ�ã���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�������Һ�Ƚ���ʱͨ��ʹ�õ�������______��
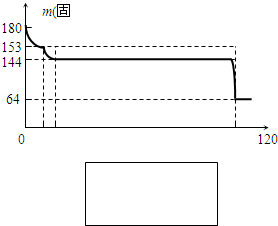
��4������AΪ��ʯ�ࣨCaSO4?2H2O���Ͳ����ᾧˮ�Ҹ���ʱҲ���ֽ�����ʣ���ʯ����120��ʱʧˮ������ʯ�ࣨ2CaSO4?H2O������ʯ����200��ʱʧˮ��������ƣ�Ϊ�ⶨ����A����ʯ��ĺ�����ij����С�����������ʵ�飺��ȡ����A 180g���������м��ȣ����ȹ����й����������¶ȱ仯��¼��ͼ��ʵ����ÿ�ζԹ������ʱ������ȴ����У�Ϊ��֤ʵ�����ľ�ȷ�ԣ�������ȴʱ�����ֹ______�������ȵ�1400��ʱ���ɵ�����ͨ��Ʒ����Һ�У�Ʒ����ɫ��д��1400��ʱ�Ļ�ѧ��Ӧ����ʽ______���۹���A����ʯ�����������=______��


�ش��������⣺
��1��������a������b����ʵ���ҽ��У������aʱ�õ��IJ���������______�����в���bʱ��ע��______��
��2��װ��a����������NH3�����ù�����ʵ�����н��У��뻭��װ��a��ʾ��ͼ��
��3���Ƚ�������ʵ�����Ƚ�����װ�ã���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�������Һ�Ƚ���ʱͨ��ʹ�õ�������______��
��4������AΪ��ʯ�ࣨCaSO4?2H2O���Ͳ����ᾧˮ�Ҹ���ʱҲ���ֽ�����ʣ���ʯ����120��ʱʧˮ������ʯ�ࣨ2CaSO4?H2O������ʯ����200��ʱʧˮ��������ƣ�Ϊ�ⶨ����A����ʯ��ĺ�����ij����С�����������ʵ�飺��ȡ����A 180g���������м��ȣ����ȹ����й����������¶ȱ仯��¼��ͼ��ʵ����ÿ�ζԹ������ʱ������ȴ����У�Ϊ��֤ʵ�����ľ�ȷ�ԣ�������ȴʱ�����ֹ______�������ȵ�1400��ʱ���ɵ�����ͨ��Ʒ����Һ�У�Ʒ����ɫ��д��1400��ʱ�Ļ�ѧ��Ӧ����ʽ______���۹���A����ʯ�����������=______��


��1������aʵ�ֹ����Һ�����ķ����ǹ��ˣ��õ��IJ���������©�������������ձ��������������ֽ⣬�����ڽᾧʱӦ���õ��������ᾧ���ʴ�Ϊ��©�������������ձ������������ᾧ��
��2������Ͱ�������Ӧ��Ϊ��ֹ��Ӧ����Ѹ�ٻ����������Σ�գ�Ҫ���÷�����װ�ã��ʴ�Ϊ��
����ͼ�������ܷ�ֹ����������װ�ã���
��3����ʵ������Һ�Ƚ�����װ���������ܣ��¿ڽ�ˮ�˿ڴ�˭�������ˮ�������෴���ʴ�Ϊ�������ܣ�
��4���ٶ�ʧˮ��ľ��������ȴʱ�����ֹ��ˮ������ᵼ�½����������������ʹƷ����Һ��ɫ�����Լ��ȵ�1400��ʱ������Ʒֽ�����˶�������
2CaSO4
| ||
CaSO4?2H20
| ||
172 36
m 180g-144g
| 172 |
| m |
| 36 |
| 180g-144g |
��ã�m=172g�����Թ���A����ʯ�����������=
| 172g |
| 180g |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ