��Ŀ����
��1����ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺

�ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ______mol?L-1����ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����______��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl-����Ŀ D��Һ���ܶ�
��2��ʵ��������480mL0.08mol/LNa2CO3��Һ�ش���������
��Ӧ��������ƽ��ȡʮˮ̼���ƾ���______g
�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ���ƽƽ��ʱ����ʵ�ʳ�����̼���ƾ�����______g��1g���������룩
��������ƿ����һ�����ʵ���Ũ�ȵ���Һ��������ƿ������______
A������ġ���B��ƿ����©ˮ��C���������Ƶ���Һ��ϴ������D���������Ҫ��
����ʵ���������������Һ��Ũ���ǡ�ƫ�ߡ�����ƫ�͡����ǡ����䡱��
A����ˮʱԽ���̶���______��
B�����ǽ�ϴ��Һ��������ƿ______��
C������ƿ�ڱڸ���ˮ���δ���ﴦ��______��
D���ܽ��û����ȴ����ж���______��
��3����ȡ����Fe2O3��ĩ������ɫ�������������ᣬ��Ӧ�����ӷ���ʽΪ______����Ӧ��õ���ɫ��FeCl3��Һ���ô���Һ������ʵ�飺
��ȡ������Һ�����Թ��У�����NaOH��Һ�������к��ɫ�������ɣ���Ӧ�����ӷ���ʽΪ______��
����С�ձ��м���25mL����ˮ�����������ں����ˮ�м���2mL FeCl3������Һ�������������Һ��______ɫ�������Ƶ�Fe��OH��3���壮
����ȡһС�ձ�����25mL����ˮ�����ձ����ټ���2mL FeCl3������Һ�����Ⱥ����ձ�����żף���ʢ��Fe��OH��3������ձ�������ң�һ����ð������ֱ��ü���������ձ��е�Һ�壬���Կ���______������ң��ձ��л���������ЧӦ����ʵ���������______��
| 1000mL��1.19g?cm -3��36.5% |
| 36.5mol/L |
�������ʵ����ʵ���Ũ��Ϊ
| 11.9mol |
| 1L |
��A��n=CV����������Һ����йأ���A����
B����Һ��Ũ���Ǿ�һ�ȶ��ģ�����ȡ��Һ������أ���B��ȷ��
C��N=nNA=CVNA����������Һ����йأ���C����
D����Һ���ܶ��Ǿ�һ�ģ���������ȡ��Һ������أ���D��ȷ��
��ѡ��BD��
��2������������Һ�����Ϊ480ml��������ƿ�Ĺ��û��480ml��ֻ��ѡ��500ml��Na2CO3�����ʵ���n=cV=0.5L��0.08mol?L-1=0.04mol��Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO3?10H2O������0.04mol��286g/mol=11.4g���ʴ�Ϊ��11.4��
�������̵�����=���̵�����+�����������֪����������=��������+�����������������������=��������-����������������������=11g-0.4g=10.6g���ʴ�Ϊ��10.6��
��������ƿ����һ�����ʵ���Ũ�ȵ���Һ����ʹ��ǰҪ�ȼ���Ƿ�©ˮ������ƿ�����۸������ʵ����û��Ӱ�죬�������������Ƶ���Һ��ϴ���ģ��ᵼ�����ƫ�ʴ�Ϊ��B��
��A����ˮʱԽ���̶��ߣ���Һ�����ƫ��Ũ��ƫС���ʴ�Ϊ��ƫ�ͣ�
B�����ǽ�ϴ��Һ��������ƿ�����ʵ�����ƫС��Ũ��ƫС���ʴ�Ϊ��ƫ�ͣ�
C������ƿ�ڱڸ���ˮ���δ���ﴦ������Һ��������䣬Ũ�Ȳ��䣬�ʴ�Ϊ�����䣻
D���ܽ��û����ȴ����ж��ݣ���Һ��ȴ�����ᵼ����Һ�����ƫС��Ũ��ƫ�ʴ�Ϊ��ƫ�ߣ�
��3����Fe2O3��ĩ�����������ᣬ�����ķ�Ӧ��Fe2O3 +6HCl=2FeCl3 +3H2O�����ӷ���ʽΪ��Fe2O3 +6H+=2Fe3++3H2O���ʴ�Ϊ��Fe2O3 +6H+=2Fe3++3H2O��
��FeCl3��Һ�е���NaOH��Һ��������Ӧ��Fe3++3OH-=Fe��OH��3�����ɹ۲쵽�к��ɫ����Fe��OH��3���ɣ��ʴ�Ϊ��Fe3++3 OH-=Fe��OH��3����
�����ˮ�е��뼸��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ������������壬�ʴ�Ϊ����֣�
��������ж����ЧӦ������Һû�ж����ЧӦ���ʴ�Ϊ���ң���Һ�ͽ��壮

 ��У����ϵ�д�
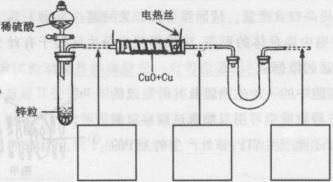
��У����ϵ�д���������ͭ��ͭ����ɵĻ���ijͬѧ������ͼ��ʾװ�ã�ͨ���ⶨ�����������ʵ��ǰ��U�������仯��ȷ�������������ͭ������������
 |
�ش��������⣺
(1)U�ι��п��Լ���������� (�����)��
A��Ũ![]() B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
(2)���в��谴ʵ�����˳��ӦΪ (����ĸ)��
a��ֹͣͨ������b������˿ͨ�磻c��ͨ��������d��װ�������Լ�飻e������˿ֹͣͨ�硣
| ʵ��Ŀ�ģ����� ʵ��ԭ�������� ʵ��������ҩƷ������ ʵ��װ�ã����� ʵ�����ݴ��������� ʵ�������������� ʵ���������ۣ����� |
(3)Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�
(4)ʵ�������ͬѧ������ʦ��ʵ�鱨����Ҫ��
Ŀ��ͼ(������������)���������ʵ�鱨���дҪ��
�Դ˷ݱ����������ۣ�������������������д��������������������Ŀո���д����ȱ��Ŀ ��
(5)��ʦ����ʵ�鱨���ָ�����ı�ʵ��ԭ��������
�Ƴ����Ӽ���ʵ�鷽�������û�ѧ����ʽ��ʾ�����
���·����ķ�Ӧԭ�� ���÷�����ⶨ������ ��
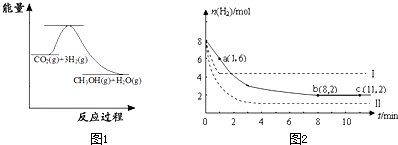
 ��2011?��������ģ���̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�
��2011?��������ģ���̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�
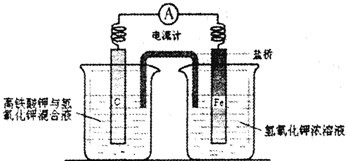

 ��3��Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�
��3��Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�