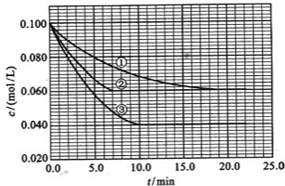
��Ŀ����
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ�����У�T��������������������������ȡ���ش��������⣺
��1��T��ԭ�ӽṹʾ��ͼΪ_______________________��
��2��Ԫ�صķǽ����ԣ�ԭ�ӵĵõ�����������Q_____________W���ǿ�ڡ������ڡ�����
��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
��4��ԭ��������R��1��Ԫ�ص�һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ��____________________________________________________��
��5��R�ж�����������м���Է���������С����һ�������£�2 L�ļ�������0.5 L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û�����������������R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ��_______________________________________��
��6����298 K��,Q��T�ĵ��ʸ�1 mol��ȫȼ�գ��ֱ�ų�����A kJ��b kJ����֪һ�������£�T�ĵ����ܽ�Q������������������û������������û���Ӧ����3 mol Q�ĵ��ʣ���÷�Ӧ��298 K�µĦ�H=_____________��ע���������浥�ʾ�Ϊ���ȶ����ʣ���
��1��![]() ��2������
��2������
��3��S+2H2SO4��Ũ��![]() 3SO2 ��+2H2O
3SO2 ��+2H2O
��4��2H2O2![]() 2H2O+O2��(������������)
2H2O+O2��(������������)
��5��NaNO2
(6)(3a-4b) kJ��mol-1
����������֪T����������������������ȣ���TΪAlԪ�أ���Q��R��W�ֱ�ΪC��N��S����5��N���������У���Է���������С����NO��2NO+O2====2NO2��2 L NO��0.5 L O2��Ӧ����1 L NO2��ʣ��1 L NO��NO2+NO+2NaOH====2NaNO2+H2O,�ɷ�Ӧ����ʽ��֪�����ɵĺ�������ΪNaNO2����6����ȼ����д����Ӧ���Ȼ�ѧ����ʽ�����ø�˹���ɣ�������÷�Ӧ�Ħ�H��

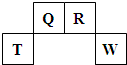 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
 ��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������
��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������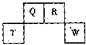 ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺