��Ŀ����
 ��1�������ʽ��з��࣬����ʶ���ʵ���ɡ��ṹ�����ʺ���;�ı��;�������÷���ķ�������ʶ�������ʣ�����ţ�����NaCl���� �ڽ���ͭ ������ ��SO2 ������
��1�������ʽ��з��࣬����ʶ���ʵ���ɡ��ṹ�����ʺ���;�ı��;�������÷���ķ�������ʶ�������ʣ�����ţ�����NaCl���� �ڽ���ͭ ������ ��SO2 ��������BaSO4 �ߴ�����
I���ܵ������
�ڢ�
�ڢ�
��II�������������ڵ���ʵ���
�٢ޢ�
�٢ޢ�
��III�����ڷǵ���ʵ���
�ܢ�
�ܢ�
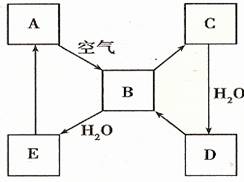
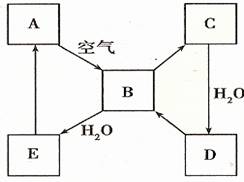
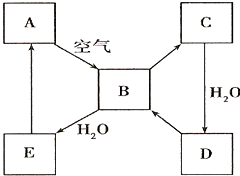
����2����ͼ��ʾij����ɫ��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����B��C����Է����������16��������D����Ҫ�Ĺ�ҵԭ�ϣ�
I������A������
��
��
��II��д��D��Ũ��Һ��Cu���ȷ�Ӧ����B�Ļ�ѧ����ʽ
Cu+H2SO4
CuSO4+SO2��+2H2O
| ||
Cu+H2SO4
CuSO4+SO2��+2H2O
��
| ||
��������1���ܵ���������к��������ƶ������ӻ����ɵ��ӣ���ˮ��Һ�������״̬���ܵ���Ļ������ǵ���ʣ���ˮ��Һ�������״̬�¶�������Ļ������Ƿǵ���ʣ�
��2��A�ܺͿ�����Ӧ����B����A�ǵ���ɫ���壬��A��S��B��SO2��B��C����Է����������16��˵��B��C�����һ��Oԭ�ӣ���C��SO3�����������ˮ��Ӧ����D��������D����Ҫ�Ĺ�ҵԭ�ϣ�����D��H2SO4��B��ˮ��Ӧ���������ᣬ����E��H2SO3��������ʵ����ʷ������
��2��A�ܺͿ�����Ӧ����B����A�ǵ���ɫ���壬��A��S��B��SO2��B��C����Է����������16��˵��B��C�����һ��Oԭ�ӣ���C��SO3�����������ˮ��Ӧ����D��������D����Ҫ�Ĺ�ҵԭ�ϣ�����D��H2SO4��B��ˮ��Ӧ���������ᣬ����E��H2SO3��������ʵ����ʷ������
����⣺��1���ܵ���������к��������ƶ������ӻ����ɵ��ӣ���ˮ��Һ�������״̬���ܵ���Ļ������ǵ���ʣ���ˮ��Һ�������״̬�¶�������Ļ������Ƿǵ���ʣ�
I��ͭ�к��������ƶ��ĵ��ӣ������к��������ƶ������ӣ�����ͭ�������ܵ��磬��ѡ�ڢۣ�
II���Ȼ��ơ����ᱵ�������ӻ������ˮ��Һ�������״̬���ܵ���������ƶ������Ӷ����磬���������ڹ��ۻ������ˮ��Һ���ܵ���������ƶ������ӵ��磬���������ǵ���ʣ���ѡ�٢ޢߣ�
III�����������ˮ��Һ�ܵ��磬�������������������Ƕ����������Զ�Ԫ�����Ƿǵ���ʣ�������ˮ��Һ�������״̬���Է��Ӵ��ڣ������������ڷǵ���ʣ���ѡ�ܢݣ�
��2��A�ܺͿ�����Ӧ����B����A�ǵ���ɫ���壬��A��S��B��SO2��B��C����Է����������16��˵��B��C�����һ��Oԭ�ӣ���C��SO3�����������ˮ��Ӧ����D��������D����Ҫ�Ĺ�ҵԭ�ϣ�����D��H2SO4��B��ˮ��Ӧ���������ᣬ����E��H2SO3��
I��ͨ�����Ϸ���֪��A�����ʣ��ʴ�Ϊ����
II���ڼ��������£�ͭ��Ũ���ᷢ��������ԭ��Ӧ��������ͭ�����������ˮ����Ӧ����ʽΪ��Cu+H2SO4
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+H2SO4
CuSO4+SO2��+2H2O��
I��ͭ�к��������ƶ��ĵ��ӣ������к��������ƶ������ӣ�����ͭ�������ܵ��磬��ѡ�ڢۣ�
II���Ȼ��ơ����ᱵ�������ӻ������ˮ��Һ�������״̬���ܵ���������ƶ������Ӷ����磬���������ڹ��ۻ������ˮ��Һ���ܵ���������ƶ������ӵ��磬���������ǵ���ʣ���ѡ�٢ޢߣ�
III�����������ˮ��Һ�ܵ��磬�������������������Ƕ����������Զ�Ԫ�����Ƿǵ���ʣ�������ˮ��Һ�������״̬���Է��Ӵ��ڣ������������ڷǵ���ʣ���ѡ�ܢݣ�
��2��A�ܺͿ�����Ӧ����B����A�ǵ���ɫ���壬��A��S��B��SO2��B��C����Է����������16��˵��B��C�����һ��Oԭ�ӣ���C��SO3�����������ˮ��Ӧ����D��������D����Ҫ�Ĺ�ҵԭ�ϣ�����D��H2SO4��B��ˮ��Ӧ���������ᣬ����E��H2SO3��
I��ͨ�����Ϸ���֪��A�����ʣ��ʴ�Ϊ����
II���ڼ��������£�ͭ��Ũ���ᷢ��������ԭ��Ӧ��������ͭ�����������ˮ����Ӧ����ʽΪ��Cu+H2SO4
| ||
�ʴ�Ϊ��Cu+H2SO4
| ||
���������⿼���˵���ʺͷǵ���ʵ��жϡ�������ƶϵ�֪ʶ�㣬���ݸ����жϵ���ʺͷǵ���ʼ��ɣ�����A����ɫ�����ʼ�ķ�Ӧ���ƶ����ʣ���ȷ���ʵ������ǽⱾ��ؼ����ѶȲ���

��ϰ��ϵ�д�
�����Ŀ