��Ŀ����
�����仯�����ڹ�ũҵ������������������Ҫ���á���ش���������

(1)��ͼ��N2��H2��Ӧ����2mol NH3�����������仯ʾ��ͼ�������ÿ����1 mol NH3�ų�����Ϊ
_____________��
(2)�����Ϊ2L���ܱ������У��������»�ѧ��Ӧ��N2(g) +3H2(g) 2NH3(g) ��H<0���õ���������
2NH3(g) ��H<0���õ���������

��ش��������⣺
�ٷ�Ӧ��ƽ�ⳣ������ʽΪ________�� K1����Դ�С��K1____(�>����=����<��)4.1�� 106��
��ʵ��l����v(H2)��ʾ�ķ�Ӧ����Ϊ_____________��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�����ݵ���____���������ĸ����
A��������N2��H2��NH3�����ʵ���Ũ��֮��Ϊ1��3��2
B��v(N2)��= 3v(H2)��
C��������ѹǿ���ֲ���
D�����������ܶȱ��ֲ���
(3)NH4Cl��Һ�����ԣ���������NH4+ˮ���Ե�ʡ���NH4Cl����ˮ(D2O)��ˮ������ӷ���ʽ��
__________________
�ٷ�Ӧ��ƽ�ⳣ������ʽΪ________�� K1����Դ�С��K1____(�>����=����<��)4.1�� 106��
��ʵ��l����v(H2)��ʾ�ķ�Ӧ����Ϊ_____________��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�����ݵ���____���������ĸ����
A��������N2��H2��NH3�����ʵ���Ũ��֮��Ϊ1��3��2
B��v(N2)��= 3v(H2)��
C��������ѹǿ���ֲ���
D�����������ܶȱ��ֲ���
(3)NH4Cl��Һ�����ԣ���������NH4+ˮ���Ե�ʡ���NH4Cl����ˮ(D2O)��ˮ������ӷ���ʽ��
__________________
(1)46.1kJ
(2)��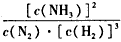 ��<����0.45 mol/(L��min)����C
��<����0.45 mol/(L��min)����C
(3)NH4++D2O NH3��HDO+D+
NH3��HDO+D+
(2)��
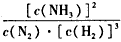 ��<����0.45 mol/(L��min)����C
��<����0.45 mol/(L��min)����C (3)NH4++D2O
 NH3��HDO+D+
NH3��HDO+D+
��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
�����Ŀ
 2NH3��g��
2NH3��g��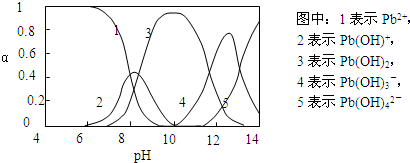
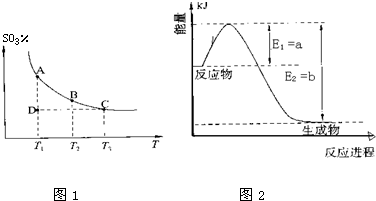
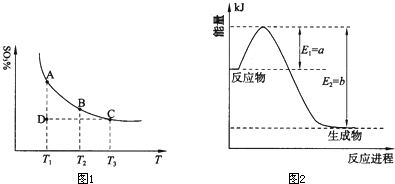

 2NH3��g������H��0���仯ѧƽ�ⳣ��K��t�Ĺ�ϵ���±�������֪K=
2NH3��g������H��0���仯ѧƽ�ⳣ��K��t�Ĺ�ϵ���±�������֪K= ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���ش��������⣺
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���ش��������⣺