��Ŀ����
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
| ������ | ��̿���ڸ��������»�ԭCuO |
| ������ | ��ⷨ����ӦΪ2Cu + H2O  Cu2O + H2���� Cu2O + H2���� |
| ������ | ���£�N2H4����ԭ����Cu(OH)2 |
��1����ҵ�ϳ��÷�����ͷ�������ȡCu2O�������÷�������ԭ���Ƿ�Ӧ���������ƣ������²��������� ��ʹCu2O���ʽ��͡�
��2����֪��2Cu(s)��1/2O2(g)=Cu2O(s) ��H = -akJ��mol-1
C(s)��1/2O2(g)=CO(g) ��H = -bkJ��mol-1
Cu(s)��1/2O2(g)=CuO(s) ��H = -ckJ��mol-1
�������ķ�Ӧ��2CuO(s)��C(s)= Cu2O(s)��CO(g)����H = kJ��mol-1��
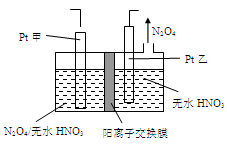
��3��������������ӽ���Ĥ���Ƶ��Һ��OH����Ũ�ȶ��Ʊ�����Cu2O��װ����ͼ��ʾ,�õ�ص���������Cu2O��ӦʽΪ ��
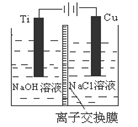
��4��������Ϊ������������Һ̬�£�N2H4����ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2�����Ʒ��Ļ�ѧ����ʽΪ ��
��5������ͬ���ܱ������У����������ַ����Ƶõ�Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺
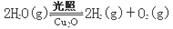 ��H >0
��H >0ˮ������Ũ�ȣ�mol/L����ʱ��t(min)�仯���±���ʾ��
| ��� | �¶� | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
����������ȷ���� ������ĸ���ţ���
A��ʵ����¶�:T2<T1
B��ʵ���ǰ20 min��ƽ����Ӧ���� v(O2)=7��10-5 mol��L-1 min-1
C��ʵ��ڱ�ʵ������õĴ�����Ч�ʸ�
��1��ͭ��Cu
��2��-(a+b-2c)kJ/mol����2c �Ca-b��2�֣� ��3�֣�
��3��2Cu��2e����2OH��=Cu2O��H2O����3�֣�
��4��4Cu(OH)2 + N2H4 2Cu2O + N2�� + 6H2O����3�֣�
2Cu2O + N2�� + 6H2O����3�֣�
��5��C��3�֣�
���������������1����̿���ڸ��������»�ԭCuO�����²���������ͭ���ʶ�ʹCu2O���ʽ��͡�
��2�����ݸ�˹���ɵ������Ȼ�ѧ����ʽ����֪�Ȼ�ѧ����ʽ�Ĺ�ϵ����������֪�Ȼ�ѧ����ʽ�ֱ�Ϊ�٢ڢ��������Ȼ�ѧ����ʽ=��+��-2���ۣ�����2CuO(s)��C(s)= Cu2O(s)��CO(g)����H ="2c-a-b" kJ��mol-1
(3)��������������Ӧ��������ɵ�������ͭ�Ļ�ѧʽ�������������ĵ缫��Ӧ����ʽΪ2Cu��2e����2OH��=Cu2O��H2O
��4��N2H4��Cu(OH)2��Ӧ�������Cu2O ��N2���ˮ���ɣ����Ի�ѧ����ʽΪ4Cu(OH)2 + N2H4 2Cu2O + N2�� + 6H2O��
2Cu2O + N2�� + 6H2O��
��5��A�� ʵ��ڢ���ȣ�ʵ��۵�ˮ��������ʼŨ����ʵ��ڵ�2������ƽ��Ũ��ȴС��2����˵��T1��T2��ƽ�������ƶ���������ӦΪ���ȷ�Ӧ������T1��T2�������¶ȣ�T2 >T1,����B�����ݷ�Ӧ���ʵĶ���ʽ��ʵ���ǰ20 min��ƽ����Ӧ����v��H2O��=7��10-5 mol��L-1 min-1 ������v(O2)=3.5��10-5 mol��L-1 min-1 ������C��ʵ�����ʵ�����ȣ��ﵽ��ƽ��״̬��ͬ��������ʱ��̣���Ӧ���ʿ죬����ʵ��ڱ�ʵ������õĴ�����Ч�ʸߣ���ȷ����ѡC��
���㣺���鷴Ӧ������жϣ���˹���ɵ�Ӧ�ã���ѧ����ʽ����д����Ӧ���ʵļ��㼰ƽ���ƶ�ԭ����Ӧ��

 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д���֪��ѧ����������ʽ���ܿ����ת������д�±��Ŀհ�:
| ��ѧ��Ӧ����ʽ(����) | ����ת����ʽ |
| �� | �ɻ�ѧ��ת��Ϊ���� |
��Pb+PbO2+2H2SO4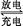 2PbSO4+2H2O 2PbSO4+2H2O | |
��CaCO3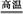 CaO+CO2�� CaO+CO2�� | |
������Ӧ������������ԭ��Ӧ����(�����)����
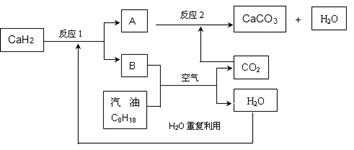
(1)�״���һ����Ҫ�Ļ�����Ʒ�������ü״��������Ʊ���ȩ����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��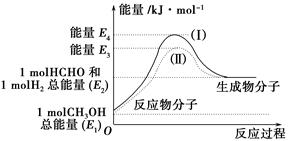
��Ӧ�����е�������ϵ
�ټ״�������ת��Ϊ��ȩ�ķ�Ӧ��________(����ȡ����ȡ�)��Ӧ��
�ڹ��̢�����̢�ķ�Ӧ���Ƿ���ͬ��____________ԭ����____________ ______________________________��
��д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ______________ _____________________��
(2)��֪����CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H����49.0 kJ��mol��1
��CH3OH(g)��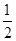 O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
����˵����ȷ����________��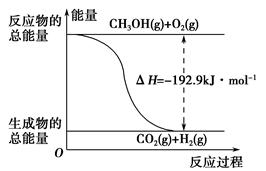
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)��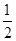 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
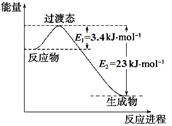
| D����Ӧ�ڵ������仯��ͼ��ʾ |
�п�Ժ�����о����о�Ա���ʽ���������ͬ�к������Ա�������PM2.5��ѧ��ɼ���Դ�ļ��ڱ仯�о����֣�����PM2.5��6����Ҫ��Դ�����У�����β����ȼú�ֱ�ռ4%��18%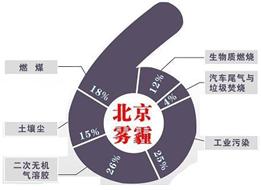
��1�����ھ�������β���ķ�ӦΪ��2NO(g)+2CO(g)
 2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
| A��װ��β������װ�õ������ų��������в��ٺ���NO��CO |
| B�����β������Ч�ʵij��÷����������¶� |
| C������ѹǿ������ƽ�����ƣ���ʵ�ʲ����п�ͨ����ѹ�ķ�ʽ����侻��Ч�� |
| D�����β������Ч�ʵ����;����ʹ�ø�Ч���� |

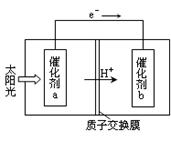
 Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ
Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ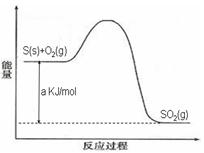
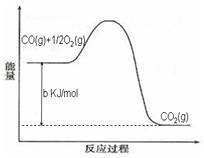
����SO2��ȥCO���Ȼ�ѧ����ʽΪ _____________________________________��
��3��NH3����ԭ�������SCR��������ĿǰӦ����㷺���������������ѳ������������Ļ�ѧ��Ӧ�ǣ�2NH3(g)+NO(g)+NO2(g)
 2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________��
2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________����4������ClO2�����������ﷴӦ�������£�

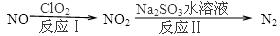
��Ӧ��Ļ�ѧ����ʽ��2NO+ClO2+H2O�TNO2+HNO3+2HCl����Ӧ������ӷ���ʽ�� ________________������11.2L N2���ɣ���״������������NO _________________ g��
��5����ҵ�����к��е�NO2�����õ�ⷨ��������NO2Ϊԭ�Ͽ���������ɫ������N2O5���Ʊ�����֮һ���Ƚ�NO2ת��ΪN2O4��Ȼ����õ�ⷨ�Ʊ� N2O5��װ����ͼ��ʾ�� Pt��Ϊ _____��������������N2O5�ĵ缫��Ӧʽ��________________��
ijʵ��С�������50 mL 1.0 mol/L�����50 mL 1.1 mol/L ����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ����(��ֽ��)��ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ����(��ֽ��)�����ձ�������ĭ���ϰ�(��Ӳֽ��)���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��Իش��������⣺
��1����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���к͡����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к���____________(�ƫ����ƫС�����䡱)��
��2�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬���˲������裬���õ��к��Ȼ�____________(�ƫ����ƫС�����䡱)��
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�____________(�ƫ����ƫС�����䡱)����ԭ����_______________________________________________��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50 mL������¼��ԭʼ����(���±�)��
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�(t2)/�� | �²�(t2��t1)/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3���кͺ���Һ�ı�����c��4.18��10��3kJ/(g����)����÷�Ӧ���к���Ϊ��H��______ __�����ݼ�������д�����кͷ�Ӧ���Ȼ�ѧ����ʽ_______________________________��
 HCO3���� H�� Ka1��H2CO3��=4.45��10��7
HCO3���� H�� Ka1��H2CO3��=4.45��10��7
 CO2(g) + 3H2(g) ����H>0
CO2(g) + 3H2(g) ����H>0
