��Ŀ����
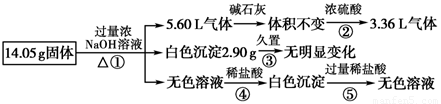
ij�������������Al����NH4��2SO4��MgCl2��FeCl2��AlCl3 �е�һ�ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ��������������ѻ���ɱ�״���µ��������
�ش��������⣺
��1��д����Ӧ�ܵ����ӷ���ʽ______��
��2����д�±��еĿհף���ע����������в����ڸóɷ֣��������������0������б�ߵı�������д��

�ش��������⣺
��1��д����Ӧ�ܵ����ӷ���ʽ______��
��2����д�±��еĿհף���ע����������в����ڸóɷ֣��������������0������б�ߵı�������д��
| �ɷ� | ���� | �ж����ݻ������������ |
| Al | ______ | ______ |
| ��NH4��2SO4 | ______ | ______ |
| MgCl2 | ______ | ______ |
| FeCl2 | ______ | ______ |
| AlCl3 | ______ | ______ |
14.05g����������������������Һ�������壬��������κͼӦ���ɵİ�����Ҳ�����ǽ�����������������Һ��Ӧ����������5.6L����ͨ����ʯ���ޱ仯��˵�������������ʯ�ҷ�Ӧ�����壬��ˮ�����Ĵ��ڣ�ͨ��Ũ���ᣬ����ʣ��3.36L���������5.6L-3.36L=2.24L����ϻ������ܴ��ڵ����ʿ�֪��һ��������������������Ʒ�Ӧ���ɰ���Ϊ2.24L��ʣ�������ֻ�������������Ϊ3.36L��˵��ԭ�������һ��������������泥�
14.05g����������������������Һ�в�����ɫ����2.9g�������ޱ仯��Fe��OH��2 �ڿ����л�ת��Ϊ���ɫFe��OH��3���ж�һ�����Ȼ�����������������������ǿ�����һ�������Ȼ�þ���������ɰ�ɫ������������þ������Ϊ2.9g��
14.05g����������������������Һ�õ���ɫ��Һ�������������� �����ɳ�����˵�����������Ȼ���������Ӧ���ɵ�ƫ�����������ᷴӦ���ɵ������������������������������ܽ��һ��֤����������������������������֪��
��1����Ӧ����ƫ������������ӷ�Ӧ���������������������ӷ���ʽAlO2-+H++H2O=Al��OH��3����
�ʴ�Ϊ��AlO2-+H++H2O=Al��OH��3����
��2��������������֪�������һ������Al����NH4��2SO4��MgCl2���ʣ�
2Al+2NaOH+2H2O=2NaAlO2+3H2��
54g 67.2L
m��Al�� 3.36L
����m��Al��=54g��
=2.7g
��NH4��2SO4+2NaOH=2Na2SO4+2H2O+2NH3��
132g 44.8L
m[��NH4��2SO4]2.24L
����m[��NH4��2SO4]=132g��
=6.6g
MgCl2+2NaOH=Mg��OH��2��+2NaCl
95g 58g
m[MgCl2]2.9g
����m[MgCl2]=95g��
=4.75g
������������֮��Ϊ2.7g+6.6g+4.75g=14.05g���ʲ���AlCl3��
�ʴ�Ϊ��
��
14.05g����������������������Һ�в�����ɫ����2.9g�������ޱ仯��Fe��OH��2 �ڿ����л�ת��Ϊ���ɫFe��OH��3���ж�һ�����Ȼ�����������������������ǿ�����һ�������Ȼ�þ���������ɰ�ɫ������������þ������Ϊ2.9g��
14.05g����������������������Һ�õ���ɫ��Һ�������������� �����ɳ�����˵�����������Ȼ���������Ӧ���ɵ�ƫ�����������ᷴӦ���ɵ������������������������������ܽ��һ��֤����������������������������֪��
��1����Ӧ����ƫ������������ӷ�Ӧ���������������������ӷ���ʽAlO2-+H++H2O=Al��OH��3����
�ʴ�Ϊ��AlO2-+H++H2O=Al��OH��3����
��2��������������֪�������һ������Al����NH4��2SO4��MgCl2���ʣ�
2Al+2NaOH+2H2O=2NaAlO2+3H2��
54g 67.2L
m��Al�� 3.36L
����m��Al��=54g��
| 3.36L |
| 67.2L |
��NH4��2SO4+2NaOH=2Na2SO4+2H2O+2NH3��
132g 44.8L
m[��NH4��2SO4]2.24L
����m[��NH4��2SO4]=132g��
| 2.24L |
| 44.8L |
MgCl2+2NaOH=Mg��OH��2��+2NaCl
95g 58g
m[MgCl2]2.9g
����m[MgCl2]=95g��
| 2.9g |
| 58g |
������������֮��Ϊ2.7g+6.6g+4.75g=14.05g���ʲ���AlCl3��
�ʴ�Ϊ��
| �ɷ� | ���� | �ж����ݻ������������ | ||
| Al | 2.7g | 2Al+2NaOH+2H2O=2NaAlO2+3H2�� 54g 67.2L m��Al�� 3.36L m��Al��=54g��
| ||
| ��NH4��2SO4 | 6.6g | ��NH4��2SO4+2NaOH=2Na2SO4+2H2O+2NH3�� 132g 44.8L m[��NH4��2SO4]2.24L m[��NH4��2SO4]=132g��
| ||
| MgCl2 | 4.75g | MgCl2+2NaOH=Mg��OH��2��+2NaCl 95g 58g m[MgCl2]2.9g m[MgCl2]=95g��
| ||
| FeCl2 | 0 | Fe��OH��2 �ڿ����л�ת��Ϊ���ɫFe��OH��3����������а�ɫ�������������Ա仯 | ||
| AlCl3 | 0 | ԭ������Al����NH4��2SO4��MgCl2�������ʵ�����֮�պõ���14.05g������һ��û��AlCl3�� |

��ϰ��ϵ�д�
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
�����Ŀ
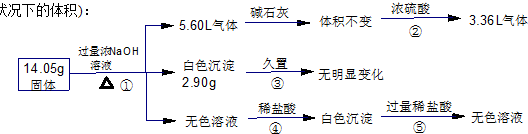
ij�������������Al����NH4��2SO4��MgCl2��FeCl2��AlCl3�е�һ�ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ��������������ѻ���ɱ�״���µ��������

����˵����ȷ���ǣ�������

����˵����ȷ���ǣ�������
| A������������һ������Al������������ȷ�� | B�����������п��ܺ���MgCl2��AlCl3 | C������������һ������MgCl2��FeCl2 | D������������һ�����У�NH4��2SO4��MgCl2 |