��Ŀ����
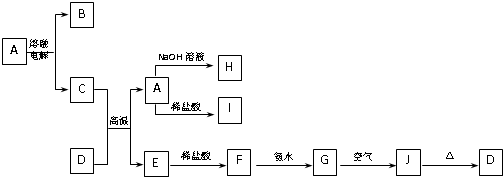
A-J����ѧ��ѧ���������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������֪A��������Ԫ����ɵĻ����D��һ�ֺ���ɫ���壬H����ɫ��Ӧ�ʻ�ɫ��
��ش��������⣺
��1��B���ʵĻ�ѧʽΪ
��2��G��J�Ļ�ѧ����ʽΪ
��3��D���������ᷴӦ������Һ�����Ե�ԭ���ǣ������ӷ���ʽ��ʾ����
��4��E��̼�γɵĺϽ��ڳ�ʪ�Ŀ����������绯ѧ��ʴ���������ĵ缫��ӦʽΪ��
��5��д��һ���������������������û���Ӧ����ʽ��
����A--J��ѡһ�ֵ�����Ϊ��Ӧ�
��������֮һ�Ǿ��д��Ե������

��ش��������⣺
��1��B���ʵĻ�ѧʽΪ
O2
O2
��H���ʵ�����Ϊƫ������
ƫ������
����2��G��J�Ļ�ѧ����ʽΪ
4Fe��OH��2+2H2O+O2�T4Fe��OH��3
4Fe��OH��2+2H2O+O2�T4Fe��OH��3
����3��D���������ᷴӦ������Һ�����Ե�ԭ���ǣ������ӷ���ʽ��ʾ����
Fe3++3H2O Fe��OH��3+3H+
Fe��OH��3+3H+
 Fe��OH��3+3H+
Fe��OH��3+3H+Fe3++3H2O Fe��OH��3+3H+
Fe��OH��3+3H+
�� Fe��OH��3+3H+
Fe��OH��3+3H+��4��E��̼�γɵĺϽ��ڳ�ʪ�Ŀ����������绯ѧ��ʴ���������ĵ缫��ӦʽΪ��
2H2O+O2+4e-=4OH-
2H2O+O2+4e-=4OH-
����5��д��һ���������������������û���Ӧ����ʽ��
3Fe+4H2O��g��
Fe3O4+4H2��
| ||
3Fe+4H2O��g��
Fe3O4+4H2��
��
| ||
����A--J��ѡһ�ֵ�����Ϊ��Ӧ�
��������֮һ�Ǿ��д��Ե������
������D��һ�ֺ���ɫ���壬ӦΪFe2O3������C��Ӧ��������A�ȿ������ᷴӦ���ֿ���NaOH��Ӧ����ת����ϵ��֪CΪAl��AΪAl2O3��EΪFe��BΪO2��HΪNaAlO2��IΪAlCl3��FΪFeCl2��GΪFe��OH��2��JΪFe��OH��3��������ʵ������Լ���ĿҪ��ɽ����⣮
����⣺D��һ�ֺ���ɫ���壬ӦΪFe2O3������C��Ӧ��������A�ȿ������ᷴӦ���ֿ���NaOH��Ӧ����ת����ϵ��֪CΪAl��AΪAl2O3��EΪFe��BΪO2��HΪNaAlO2��IΪAlCl3��FΪFeCl2��GΪFe��OH��2��JΪFe��OH��3��
��1�������Ϸ�����֪BΪO2��HΪƫ�����ƣ��ʴ�Ϊ��O2��ƫ�����ƣ�
��2��GΪFe��OH��2�����ȶ������л�ԭ�ԣ������������������������ԭ��Ӧ����Fe��OH��3����Ӧ�Ļ�ѧ����ʽΪ4Fe��OH��2+2H2O+O2�T4Fe��OH��3��
�ʴ�Ϊ��4Fe��OH��2+2H2O+O2�T4Fe��OH��3��
��3��DΪFe2O3�������ᷴӦ����FeCl3����ǿ�������Σ�ˮ������ԣ����ӷ���ʽΪFe3++3H2O Fe��OH��3+3H+��
Fe��OH��3+3H+��
�ʴ�Ϊ��Fe3++3H2O Fe��OH��3+3H+��
Fe��OH��3+3H+��
��4��Fe��̼�γɵĺϽ��ڳ�ʪ�Ŀ����������绯ѧ��ʴ��Ϊ������ʴ���������ĵ缫��ӦʽΪ2H2O+O2+4e-=4OH-���ʴ�Ϊ��2H2O+O2+4e-=4OH-��
��5��������֮һ�Ǿ��д��Ե������ӦΪFe3O4��ӦΪ����ˮ�����ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ3Fe+4H2O��g��
Fe3O4+4H2����
�ʴ�Ϊ��3Fe+4H2O��g��
Fe3O4+4H2����
��1�������Ϸ�����֪BΪO2��HΪƫ�����ƣ��ʴ�Ϊ��O2��ƫ�����ƣ�
��2��GΪFe��OH��2�����ȶ������л�ԭ�ԣ������������������������ԭ��Ӧ����Fe��OH��3����Ӧ�Ļ�ѧ����ʽΪ4Fe��OH��2+2H2O+O2�T4Fe��OH��3��
�ʴ�Ϊ��4Fe��OH��2+2H2O+O2�T4Fe��OH��3��
��3��DΪFe2O3�������ᷴӦ����FeCl3����ǿ�������Σ�ˮ������ԣ����ӷ���ʽΪFe3++3H2O
 Fe��OH��3+3H+��
Fe��OH��3+3H+���ʴ�Ϊ��Fe3++3H2O
 Fe��OH��3+3H+��
Fe��OH��3+3H+����4��Fe��̼�γɵĺϽ��ڳ�ʪ�Ŀ����������绯ѧ��ʴ��Ϊ������ʴ���������ĵ缫��ӦʽΪ2H2O+O2+4e-=4OH-���ʴ�Ϊ��2H2O+O2+4e-=4OH-��
��5��������֮һ�Ǿ��д��Ե������ӦΪFe3O4��ӦΪ����ˮ�����ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ3Fe+4H2O��g��
| ||
�ʴ�Ϊ��3Fe+4H2O��g��
| ||
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�ע���DΪͻ�ƿڣ�������ʵ�ת���ƶ��������ʣ��ι�����������ʵ�����Ϊ��������Ŀ�Ĺؼ���

��ϰ��ϵ�д�
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�
�����Ŀ



